Steps For Drawing Lewis Structures
Steps For Drawing Lewis Structures - This chemistry video provides a basic introduction into how to. Note down a skeletal structure displaying a realistic bonding pattern by means of only the element symbols. Using lewis structures to show valence electrons. Attach the terminal atoms using pairs of electrons. Find the total number of valence electrons (refer to the instructions below the pictures) In the very first step, you have to calculate the total number of valence electrons of a given molecule. Be aware there are several different strategies for constructing lewis structures. Try to satisfy the octets of the atoms by distributing the remaining valence electrons as nonbonding electrons. When constructing a lewis diagram, keep in mind the octet rule, which refers to the tendency. 2.1m views 6 years ago new ap & general chemistry video playlist. A lewis electron dot formula comprises one dot for every valence electron and an element’s symbol. You start by counting the number of electrons the atoms donate to the structure, and you need to take into account charge if you are drawing an ion. Web drawing lewis structures for molecules with one central atom: Find the total number of valence. Usually, the central atom is already known as in the case with many organic compounds containing carbon as the. How to draw electron dot structures? Breaking the octet rule ; To use the lewis structure calculator follow these steps: * hydrogen atoms are always terminal (only one bond) * put more electronegative elements in terminal positions. Arrange the atoms to show specific connections. Examples for drawing lewis structures for covalent bonds. Using lewis structures to show valence electrons. Using the periodic table to draw lewis dot structures. Draw the simple structure (skeleton structure) of the compound by connecting everything with single bonds only. Web follow these simple steps to correctly draw a lewis dot structure: Be aware there are several different strategies for constructing lewis structures. * hydrogen atoms are always terminal (only one bond) * put more electronegative elements in terminal positions. Write the correct skeletal structure for the molecule. You figure out what the connections between atoms are. Draw the simple structure (skeleton structure) of the compound by connecting everything with single bonds only. Make sure honc and the octet rule is met and form multiple bonds if necessary. Sum the valence electrons from all the atoms. Using the periodic table to draw lewis dot structures. A lewis diagram shows how the valence electrons are distributed around the. For very simple molecules and molecular ions, we can write the lewis structures by merely pairing up the unpaired electrons on the constituent atoms. Fill the octets of the atoms bonded to the central atom. Web for very simple molecules and molecular ions, we can write the lewis structures by merely pairing up the unpaired electrons on the constituent atoms.. You start by counting the number of electrons the atoms donate to the structure, and you need to take into account charge if you are drawing an ion. Stages to articulate the electron dot formula are stated beneath. 234k views 3 years ago. * hydrogen atoms are always terminal (only one bond) * put more electronegative elements in terminal positions.. Sum the valence electrons from all the atoms. Examples for drawing lewis structures for covalent bonds. Don't forget to include any positive or negative charges when determining this. Web a video tutorial for how to draw lewis structures in five steps. Place the remaining electrons on the central atoms. The video covers the basic lewis structures you'll see in an introductory chemistry class. Count the valence electrons for each atom, add them up, and add or remove electrons if there is an overall charge. Use two valence electrons to form each bond in the skeleton structure. Web drawing lewis structures for molecules with one central atom: Drawing lewis structures. This is probably the most difficult step in drawing lewis structures because we must pick one or multiple atoms to be the central connector to terminal (outside) atoms. Place the remaining electrons on the central atoms. Determine the central atom and connect the other atoms to it. Using the periodic table to draw lewis dot structures. Attach the terminal atoms. You count the valence electrons. In the very first step, you have to calculate the total number of valence electrons of a given molecule. Web in short, these are the steps you need to follow for drawing a lewis structure: Attach the terminal atoms using pairs of electrons. Determine the central atom and connect the other atoms to it. Make sure honc and the octet rule is met and form multiple bonds if necessary. Place the remaining electrons on the central atoms. Add electrons to form single bonds between the atoms. This is probably the most difficult step in drawing lewis structures because we must pick one or multiple atoms to be the central connector to terminal (outside) atoms. Web the organic chemistry tutor. Calculate the total number of valence electrons. Web to draw lewis structures. Use two valence electrons to form each bond in the skeleton structure. Stages to articulate the electron dot formula are stated beneath. Web drawing lewis structures for molecules with one central atom: To use the lewis structure calculator follow these steps:/Lewis-dot-structure-58e5390f3df78c5162b4c3db.jpg)
How to Draw a Lewis Structure

How to draw Lewis Structures a step by step tutorial Middle School
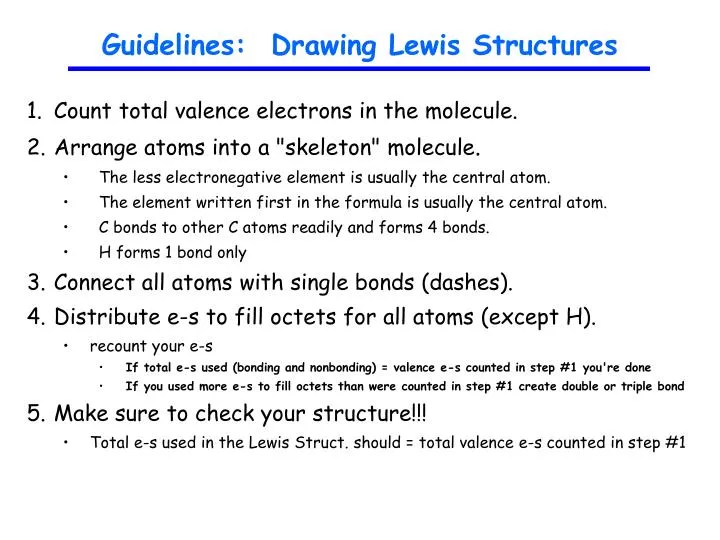
How To Draw Lewis Structures A Step By Step Tutorial

PPT Drawing Lewis structures PowerPoint Presentation, free download

How To Draw Lewis Structures A Comprehensive Guide IHSANPEDIA

3 Ways to Draw Lewis Dot Structures wikiHow

How To Draw Lewis Structures A Step By Step Tutorial

Infografik Zeichnen Sie eine LewisPunktStruktur. Becherglas Babe

How To Draw Lewis Structures A Step By Step Tutorial

How to Draw a Lewis Structure
This Chemistry Video Provides A Basic Introduction Into How To.
Web The Lewis Structure Generator That We Put In Your Hands Here Is An Excellent Tool To Obtain Structures Of More Than 400 Molecules.
How To Draw Electron Dot Structures?
Using The Periodic Table To Draw Lewis Dot Structures.
Related Post: