Sigma Bond Drawing
Sigma Bond Drawing - Sigma bonds form when the available orbital with the highest energy of each atom overlaps one another. Combining atomic orbitals, sigma and pi bonding. Web a single π bond is drawn as two electron clouds one arising from each lobe of the p orbitals. Describe the formation of covalent bonds in terms of molecular orbitals. * in a triple bond. Thus we need to leave one electron (in case of carbon double bond) to let the carbon have the second bond as a. Various bond parameters such as bond length, bond angle, and bond enthalpy depend on the way the overlapping of atomic orbital takes place. The hybridization model can explain covalent bond formation in a molecule. Web when orbitals approach each other in a head to head fashion, the resulting covalent bonds are called sigma bonds. Web sigma and pi bonds are an aspect of valence bond theory and molecular orbital theory that explains the existence of double and triple bonds. As we have been discussing how to use lewis structures to depict the bonding in organic compounds, we have been very vague so far in our language about the actual nature of the chemical bonds themselves. Web this is called a sigma bond, where the overlap is along the same axis as if you connected the two molecules. This chemistry. Covalent bonds are formed by overlapping atomic orbitals, resulting in sigma and pi bonds. Web #charusscienceworld drawing of sigma and pi bonds formation in n2 molecule As illustrations, consider the bonds that have already been studied. Web three sigma bonds are formed from each carbon atom for a total of six sigma bonds in the molecule. * all the atoms. Web this is called a sigma bond, where the overlap is along the same axis as if you connected the two molecules. Likewise, a triple bond consists of one sigma bond and two pi bonds. This is a pi bond. Sigma (σ) and pi (π). With this information, you can easily count sigma and pi. Sigma and pi bonds are the two types of covalent bonds found in molecules and compounds. 1.1m views 6 years ago new ap & general chemistry video playlist. How to count sigma and pi bonds? Web this is called a sigma bond, where the overlap is along the same axis as if you connected the two molecules. This chemistry video. Sigma bonding is most simply defined for diatomic molecules using the language and tools of. And a hybridized orbital cannot be involved in a pi bond. Web the figure below illustrates the sigma and pi bonds in an ethylene molecule (\(c_2h_4\)). Describe the formation of covalent bonds in terms of molecular orbitals. Web three sigma bonds are formed from each. Every single bond is 1 sigma bond, every double bond has 1 sigma and 1 pi bond, and every triple bond has 1 sigma bond and 2 pi bonds. The pi bond is the second bond of the double bonds between the carbon atoms, and is shown as an elongated green lobe that extends both above and below the plane. Various bond parameters such as bond length, bond angle, and bond enthalpy depend on the way the overlapping of atomic orbital takes place. Sigma bonding is most simply defined for diatomic molecules using the language and tools of. Web three sigma bonds are formed from each carbon atom for a total of six sigma bonds in the molecule. 9.4 sigma. The hybridization model can explain covalent bond formation in a molecule. The two clouds of electrons in a π bond represent one bond containing two electrons. * all the atoms have linear geometry. As we have been discussing how to use lewis structures to depict the bonding in organic compounds, we have been very vague so far in our language. * all the atoms have linear geometry. And a hybridized orbital cannot be involved in a pi bond. The pi bond is the second bond of the double bonds between the carbon atoms, and is shown as an elongated green lobe that extends both above and below the plane of the molecule. As we have been discussing how to use. Draw simple molecular orbital diagrams (e.g., for the h 2 molecule) showing the formation of. Π bonds are only found within double and triple bonds. Web sigma and pi bonds are an aspect of valence bond theory and molecular orbital theory that explains the existence of double and triple bonds. A sigma bond is the first bond between two atoms,. The two clouds of electrons in a π bond represent one bond containing two electrons. As we have been discussing how to use lewis structures to depict the bonding in organic compounds, we have been very vague so far in our language about the actual nature of the chemical bonds themselves. Note that every single bond consists of one sigma bond, and that the double bond is made of one sigma bond and one pi bond. Web how do you count sigma and pi bonds? Web sigma and pi bonds are an aspect of valence bond theory and molecular orbital theory that explains the existence of double and triple bonds. This is the strongest form of covalent bonds, and this'll be a good basis for discussion maybe in the next video when we talk a little bit about pi bonds. Over here, you connect the two molecules, the overlap is on that same axis. This chemistry video tutorial provides a basic introduction into sigma and pi bonds. Web three sigma bonds are formed from each carbon atom for a total of six sigma bonds in the molecule. * in a triple bond. Account for differences in bond length and strength in terms of the efficiency with which atomic orbitals overlap. A sigma bond is the first bond between two atoms, and is the strongest sort of covalent bond. Web drawing lewis structures with multiple bonds. This is a pi bond. With this information, you can easily count sigma and pi. Web the two p orbitals of each carbon overlap to make two π bonds.[Solved] sketch sigma and pi bond from p orbital Course Hero
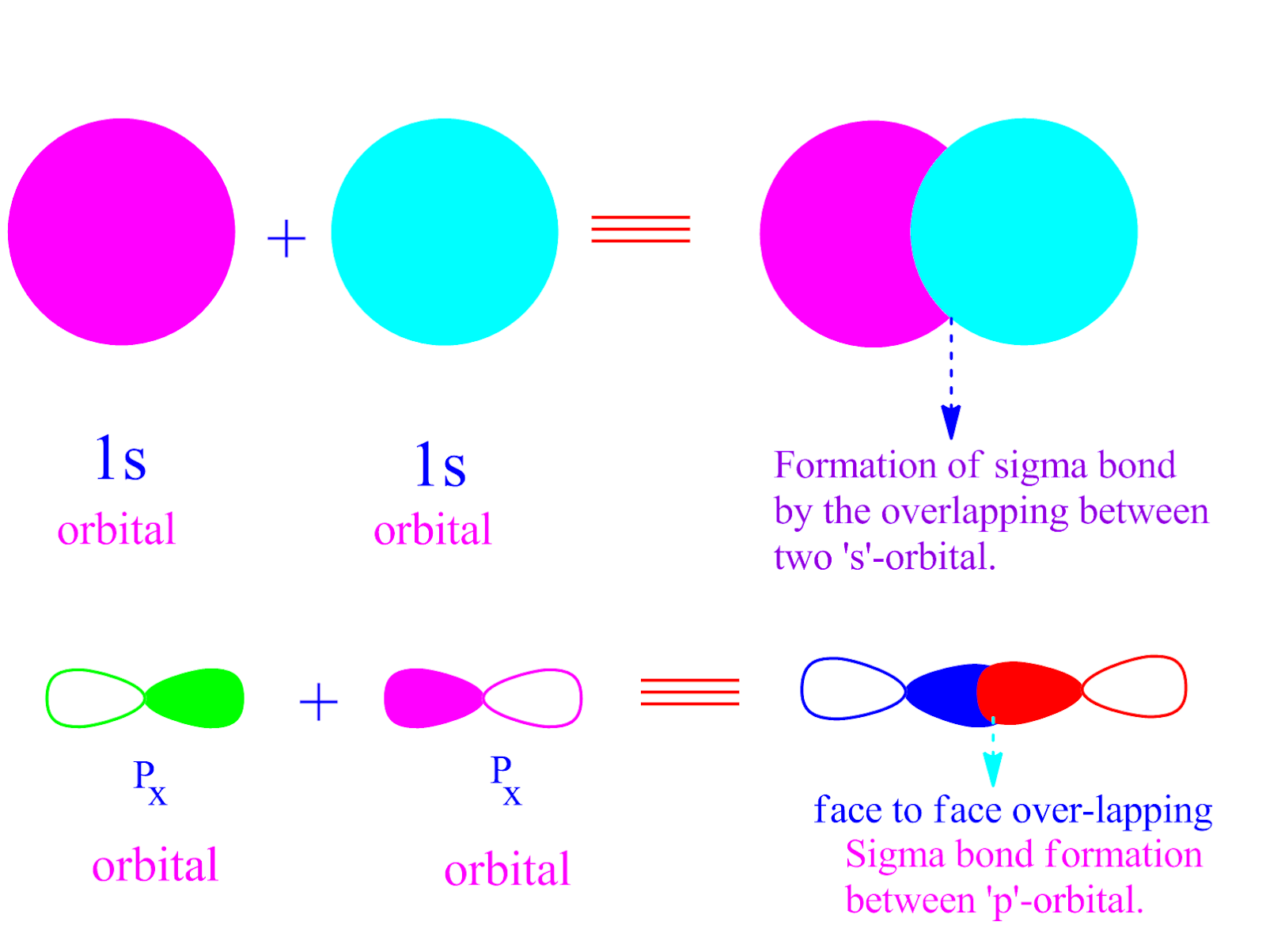
Why are sigma bond more stronger than pi bond ? PG.CHEMEASY
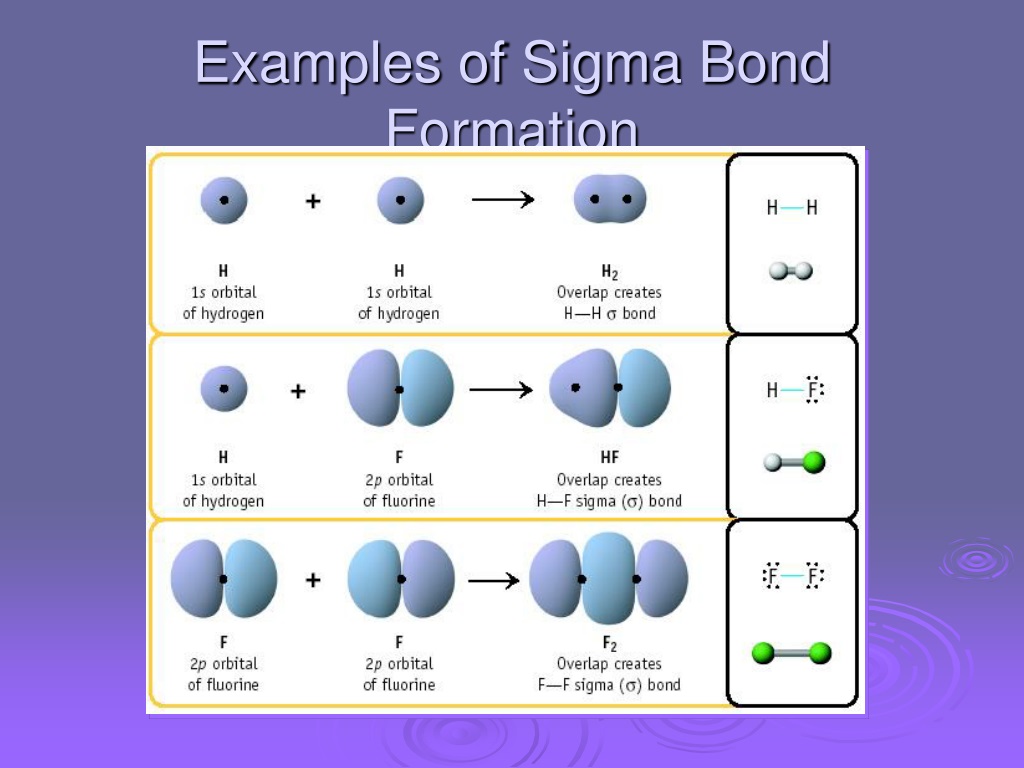
Sigma bond gilitmetro
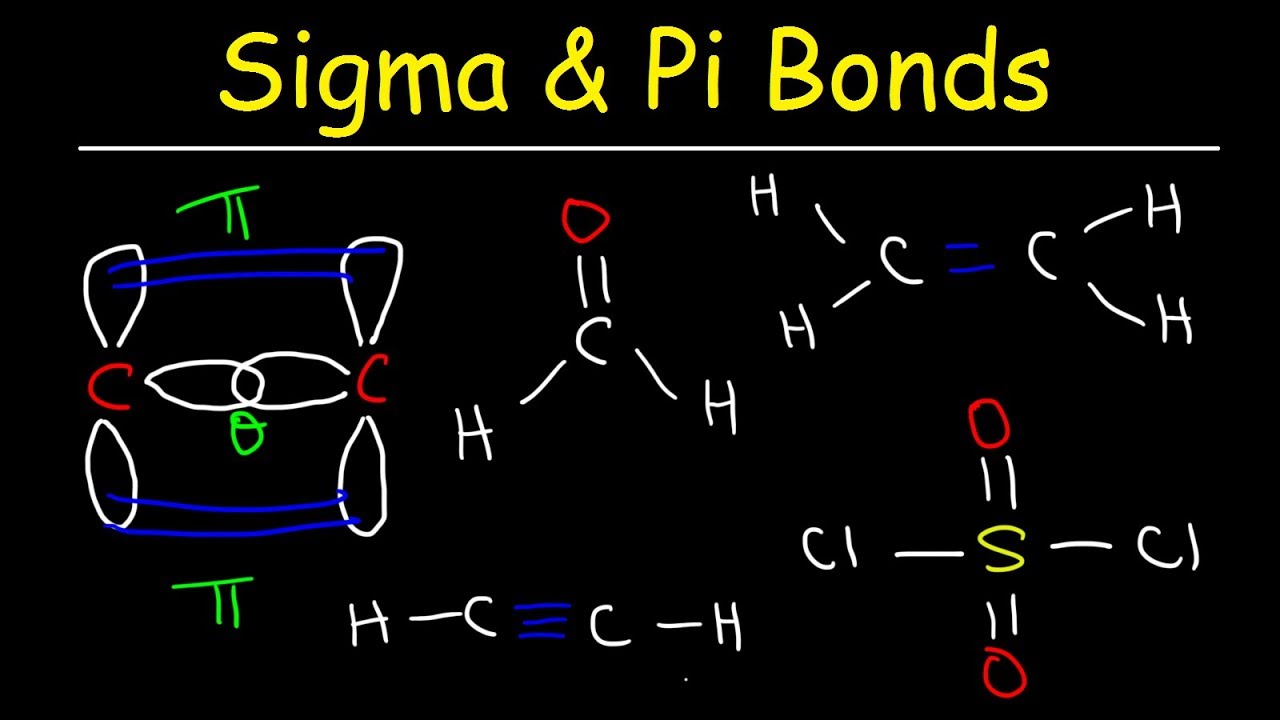
Sigma and Pi Bonds Explained, Basic Introduction, Chemistry Inflation
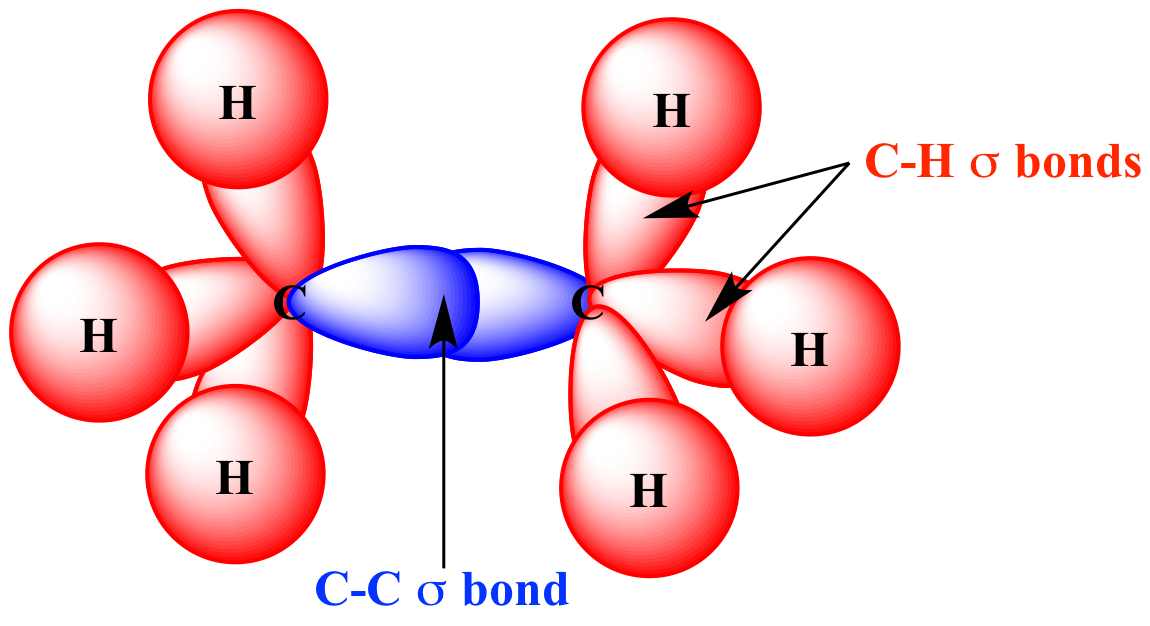
Illustrated Glossary of Organic Chemistry Sigma bond (σ bond)

Sigma and Pi Bonds Brilliant Math & Science Wiki
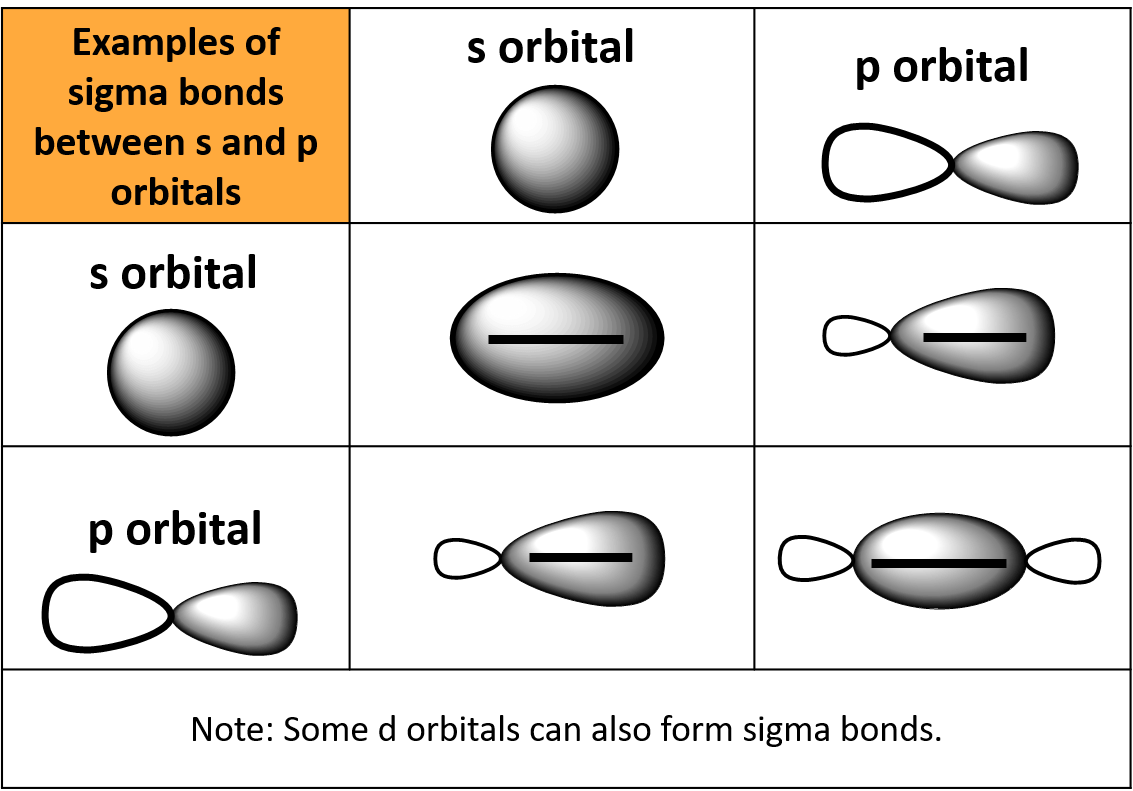
Sigma and Pi Bonds — Definition & Overview Expii

Sigma (δ) and Pi ((π) Bond (ALevel) ChemistryStudent

Draw sigma and pi bonds YouTube

8 Drawing Molecular Orbital Diagrams — Flux Science
Sigma And Pi Bonds Play A Crucial Role In Understanding The Structure, Stability, And Reactivity Of A Wide Range Of Chemical Species.
And A Hybridized Orbital Cannot Be Involved In A Pi Bond.
Likewise, A Triple Bond Consists Of One Sigma Bond And Two Pi Bonds.
The Key Parameters Of The Sp Hybridization And Triple Bond:
Related Post: