Rules For Drawing Lewis Dot Structures
Rules For Drawing Lewis Dot Structures - Single, double or triple) is critically important for predicting the chemical and physical properties of the covalent compound. Below is a table that coordinates the group number to the valence electrons. So let's say we wanted to draw the dot structure for this molecule, so silicon tetrafluoride. Combining atoms to form covalent molecules can be accomplished following five easy steps: In any molecule or ion with the general formula ab n , the unique atom (a) is in the center and all of the b atoms are attached to a. Shared pairs of electrons are drawn as lines between atoms, while. The following procedure can be used to construct lewis electron structures for more complex molecules and ions: Web to draw the lewis structure, you will need to know the total number of valence electrons present. 1) determine which atoms are connected to each other. Web how to draw lewis structures. Web here are the steps to draw a lewis structure. Know the exceptions to the octet rule. For ions, the charge must be taken into account. The lewis dot structure for formaldehyde. Web write lewis structures for molecules and ions. A lewis structure is a graphic representation of the electron distribution around atoms. The diagram is also called a lewis dot diagram, lewis dot formula, or electron dot diagram. Stages to articulate the electron dot formula are stated beneath. Be sure to have the correct number of electrons. In any molecule or ion with the general formula ab n ,. In any molecule or ion with the general formula ab n , the unique atom (a) is in the center and all of the b atoms are attached to a. A lewis structure is a diagram that shows the chemical bonds between atoms in a molecule and the valence electrons or lone pairs of electrons. Only give reasonable results for. Draw lewis structures depicting the bonding in simple molecules. You start by counting the number of electrons the atoms donate to the structure, and you need to take into account charge if you are drawing an ion. Web drawing lewis dot structures and resonance structures. Determine the total number of valence electrons. Combining atoms to form covalent molecules can be. Write lewis symbols for neutral atoms and ions. Web write lewis structures for molecules and ions. Use two valence electrons to form each bond in the skeleton structure. Draw the atoms on paper and put dots around them to represent valence electrons of the atom. The structure (what atom is bound to what atom) for covalent compounds is not always. Determine the total number of valence electrons in the molecule or ion. Remember that lewis dot structures. Lewis structures are structural formulas for molecules and polyatomic ions that represent all valence electrons. In any molecule or ion with the general formula ab n , the unique atom (a) is in the center and all of the b atoms are attached. Web general rules for drawing lewis structures. 3) place two electrons between each atom in place of the bonds. Web rules for drawing lewis dot structures. Lewis structures are structural formulas for molecules and polyatomic ions that represent all valence electrons. These types of bonds involve the metal donating its valence electron (s) to a nonmetal, forming an ionic compound. If a metal and a nonmetal interact, then an ionic bond will result. Web rules for drawing lewis dot structures: Web to draw the lewis structure, you will need to know the total number of valence electrons present. Only give reasonable results for covalent compounds and polyatomic ions of the main group (s and p block) elements, can not predict. Lewis structures and the octet rule. In any molecule or ion with the general formula ab n , the unique atom (a) is in the center and all of the b atoms are attached to a. A lewis electron dot formula comprises one dot for every valence electron and an element’s symbol. Basic concepts of chemical bonding. The number of. Combining atoms to form covalent molecules can be accomplished following five easy steps: Web here are the steps to draw a lewis structure. Web there are multiple ways to draw lewis dot structures. Web rules for drawing lewis dot structures: The diagram is also called a lewis dot diagram, lewis dot formula, or electron dot diagram. Web rules for drawing lewis structures complete the lewis structures in the table on the datasheet. Web rules for drawing lewis dot structures: You start by counting the number of electrons the atoms donate to the structure, and you need to take into account charge if you are drawing an ion. A lewis diagram shows how the valence electrons are distributed around the atoms in a molecule. Web drawing lewis dot structures and resonance structures. Lewis structures and the octet rule. Basic concepts of chemical bonding. These types of bonds involve the metal donating its valence electron (s) to a nonmetal, forming an ionic compound. Try to satisfy the octets of the atoms by distributing the remaining valence electrons as nonbonding electrons. Web general rules for drawing lewis structures. A lewis electron dot formula comprises one dot for every valence electron and an element’s symbol. Shared pairs of electrons are drawn as lines between atoms, while. However, the bonding (what is bound to what and the order of the bonds: Web how to draw lewis structures. 4) add the rest of the available valence electrons to complete the octet of the surrounding atoms. Determine the total number of valence electrons.
Comment Dessiner Une Structure De Lewis Diverses Structures
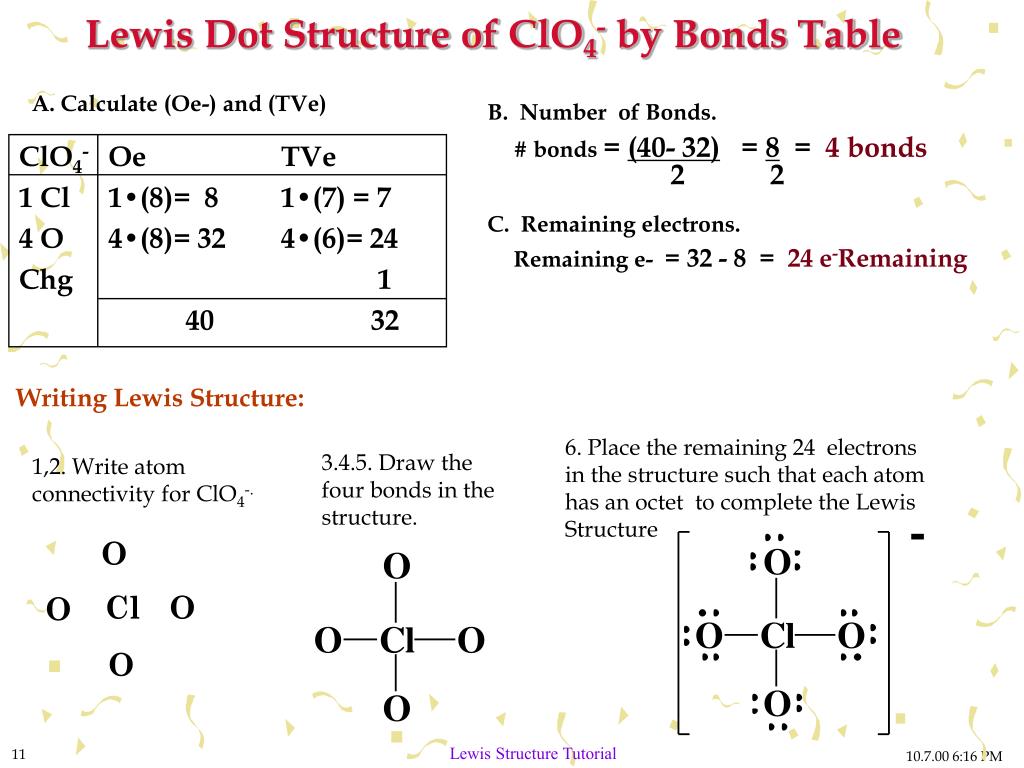
Lewis Electron Dot Structure Calculator
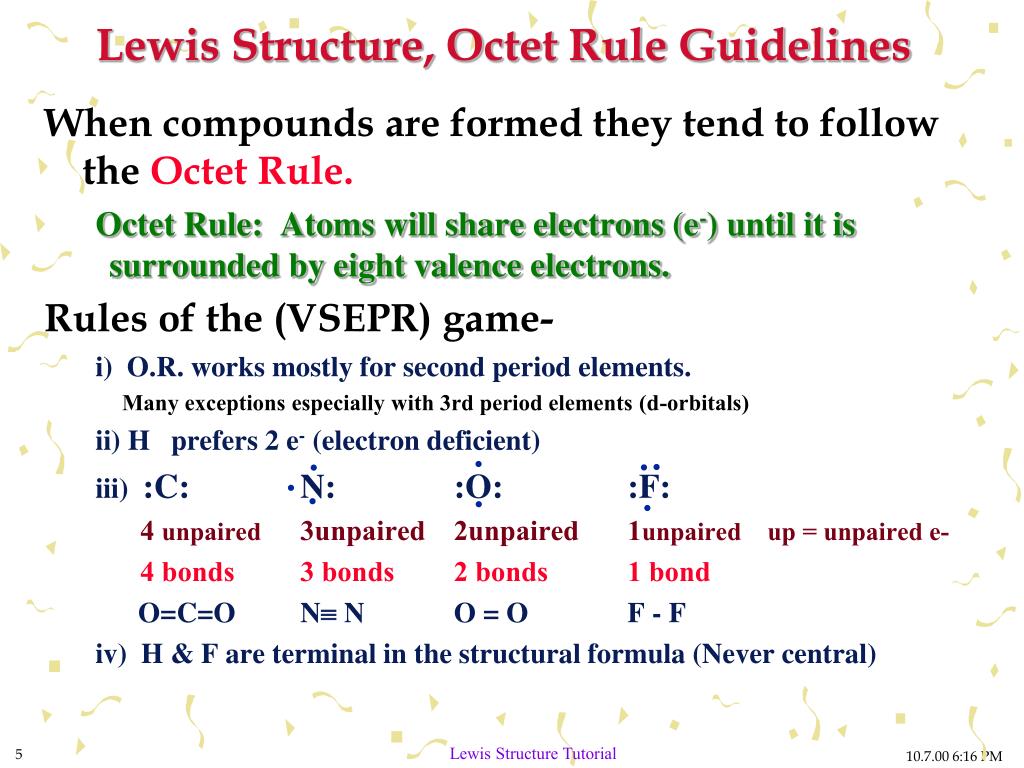
PPT Drawing Lewis Structures A Tutorial on Writing Lewis Dot
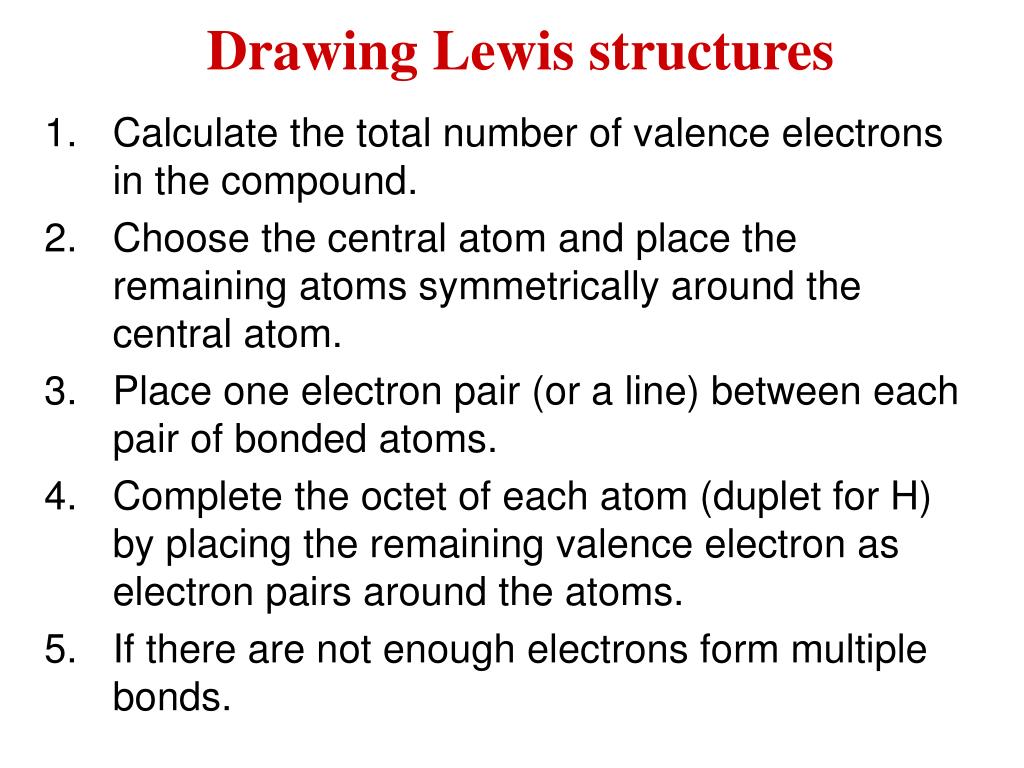
PPT Drawing Lewis structures PowerPoint Presentation, free download

How to Draw a Lewis Structure

How to draw Lewis Structures a step by step tutorial Middle School
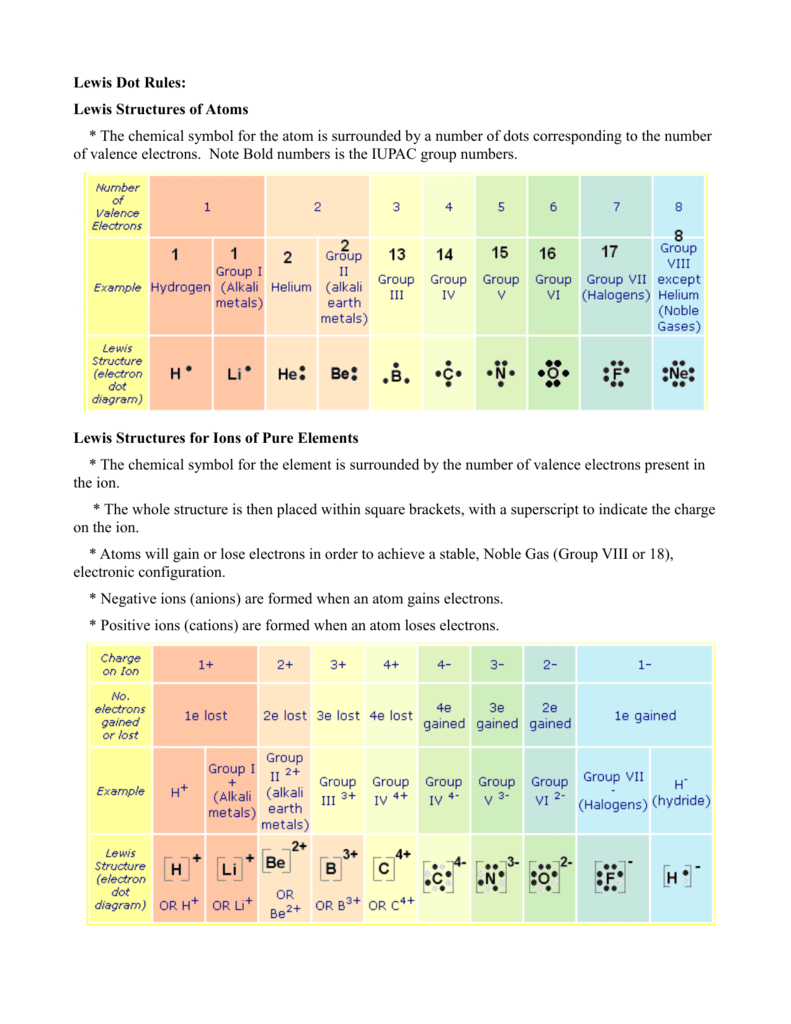
Lewis Dot Rules Lewis Structures of Atoms * The chemical symbol

How to Draw a Lewis Structure

Lewis Dot Structure Calculator macfasr
How To Draw Lewis Dot Diagrams Simplereality27
Note Down A Skeletal Structure Displaying A Realistic Bonding Pattern By Means Of Only The Element Symbols.
The Structure (What Atom Is Bound To What Atom) For Covalent Compounds Is Not Always Easy To Infer From The Molecular Formula.
Determine The Total Number Of Valence Electrons In The Molecule Or Ion.
The First Thing We Would Need To Do Is To Find The Total Number Of Valence Electrons.
Related Post: