Polarity Drawing
Polarity Drawing - Web polarity symbols are a notation for electrical polarity, found on devices that use direct current (dc) power, when this is or may be provided from an alternating current (ac) source via an ac adapter. See how the molecule behaves in an electric field. If individual dipoles within a molecule cancel, there is no net dipole. Find out by adding single, double or triple bonds and lone pairs to the central atom. Assess the polarity of a molecule based on its bonding and structure. Based on the lewis diagrams, which of the following correctly compares the polarities of compounds 1 and 2? Graph functions, plot points, visualize algebraic equations, add sliders, animate graphs, and more. Web we recommend using the latest version of chrome, firefox, safari, or edge. The polarity of a bond—the extent to which it is polar—is determined largely by the relative electronegativities of the bonded atoms. Experiment with different molecules and electric fields and observe the results. Based on the lewis diagrams, which of the following correctly compares the polarities of compounds 1 and 2? Recognize bond characteristics of covalent compounds: It explains how to indicate the polarity of a bond and of a. Graph functions, plot points, visualize algebraic equations, add sliders, animate graphs, and more. Web explore math with our beautiful, free online graphing calculator. It explains how to indicate the polarity of a bond and of a. Covalent bonding and simple molecular compounds. There is an abundance of experimental evidence to that effect—from their physical. Determine if a molecule is polar or nonpolar. Explain the concepts of polar covalent bonds and molecular polarity. If individual dipoles within a molecule cancel, there is no net dipole. See how the molecule behaves in an electric field. Define electronegativity and assess the polarity of covalent bonds. Graph functions, plot points, visualize algebraic equations, add sliders, animate graphs, and more. The difference in electronegativity across a molecule can generate electric dipole moments. Use electronegativity values to predict bond polarity. Two different compounds have the chemical formula xef a 2 cl a 2. It explains how to indicate the polarity of a bond and of a. Graph functions, plot points, visualize algebraic equations, add sliders, animate graphs, and more. Assess the polarity of a molecule based on its bonding and structure. Determine the polarity of molecules using net molecular dipoles. Thus there is a direct correlation between electronegativity and bond. Web we recommend using the latest version of chrome, firefox, safari, or edge. Experiment with different molecules and electric fields and observe the results. Two different compounds have the chemical formula xef a 2 cl a 2. Then, compare the model to real molecules! Predict the structures of small molecules using valence shell electron pair repulsion (vsepr) theory. In our previous work we learned why atoms form covalent bonds and how to draw the resulting organization of atoms. Bond length and bond polarity. Explore molecule shapes by building molecules in 3d! Web university of kansas. Predict the structures of small molecules using valence shell electron pair repulsion (vsepr) theory. The structural formula editor is surround by three toolbars which contain the tools you can use in the editor. If individual dipoles within a molecule cancel, there is no net dipole. Web polarity symbols are a notation for electrical polarity, found on. Web to determine if this molecule is polar, we draw the molecular structure. Determine the shape of simple molecules. Explain the concepts of polar covalent bonds and molecular polarity. In our previous work we learned why atoms form covalent bonds and how to draw the resulting organization of atoms. We also use lewis symbols to indicate the formation of covalent. It explains how to indicate the polarity of a bond and of a. Determine if a molecule is polar or nonpolar. Dipole moments are vector quantities, and by convention point from a more positive region of charge to a more negative region. Web describe the formation of covalent bonds. Web draw out the line structure of the molecule with a. Given a pair of compounds, predict which would have a higher melting or boiling point. Thus there is a direct correlation between electronegativity and bond. By the end of this section, you will be able to: Two different compounds have the chemical formula xef a 2 cl a 2. In our previous work we learned why atoms form covalent bonds. How does molecule shape change with different numbers of bonds and electron pairs? Web we recommend using the latest version of chrome, firefox, safari, or edge. Experiment with different molecules and electric fields and observe the results. The structural formula editor is surround by three toolbars which contain the tools you can use in the editor. Web now that we understand how to draw dot structures and we know how to predict the shapes of molecules, let's use those skills to analyze the polarity of molecules, using what's called the dipole moment. Web discover how the shape and electronegativity of molecules determine their polarity in this phet simulation. Change the bond angle to see how shape affects polarity. Define electronegativity and assess the polarity of covalent bonds. Web explore math with our beautiful, free online graphing calculator. Determine if a molecule is polar or nonpolar. Two different compounds have the chemical formula xef a 2 cl a 2. Given a pair of compounds, predict which would have a higher melting or boiling point. If individual dipoles within a molecule cancel, there is no net dipole. Graph functions, plot points, visualize algebraic equations, add sliders, animate graphs, and more. Web to determine if this molecule is polar, we draw the molecular structure. The ability of an atom in a molecule to attract shared electrons is called electronegativity.
What is a Polar Covalent Bond? ChemTalk
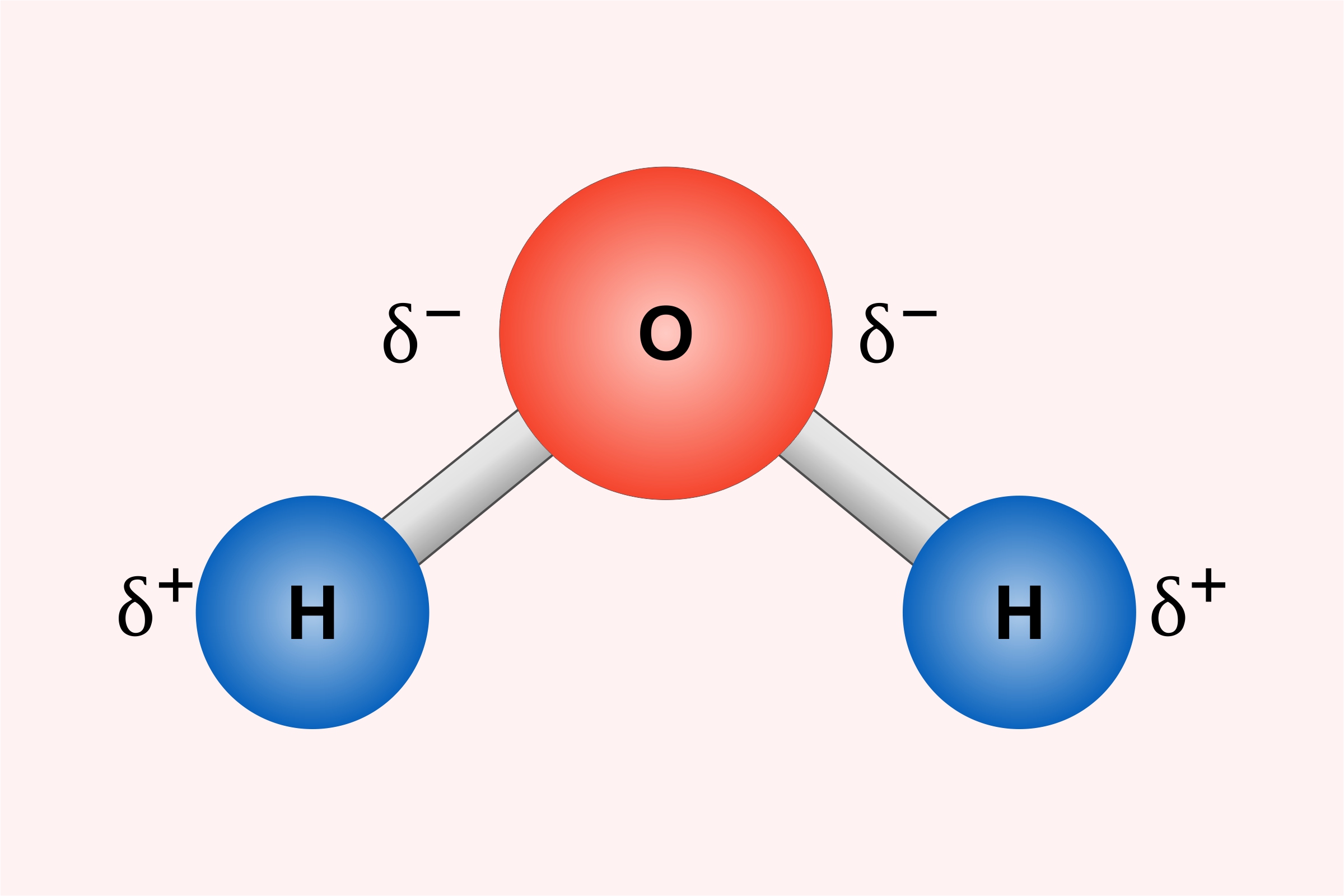
What does the term polarity mean in biology? Biology Questionnaire
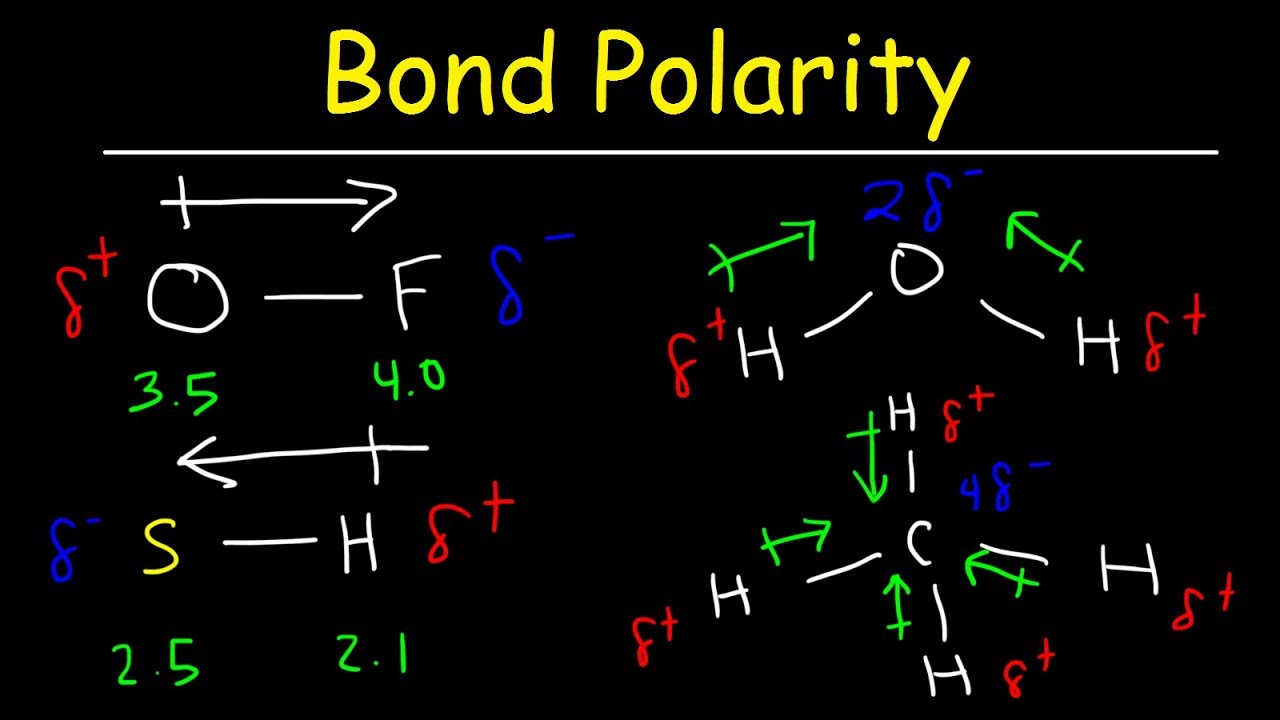
Bond Polarity, Electronegativity and Dipole Moment Chemistry Practice
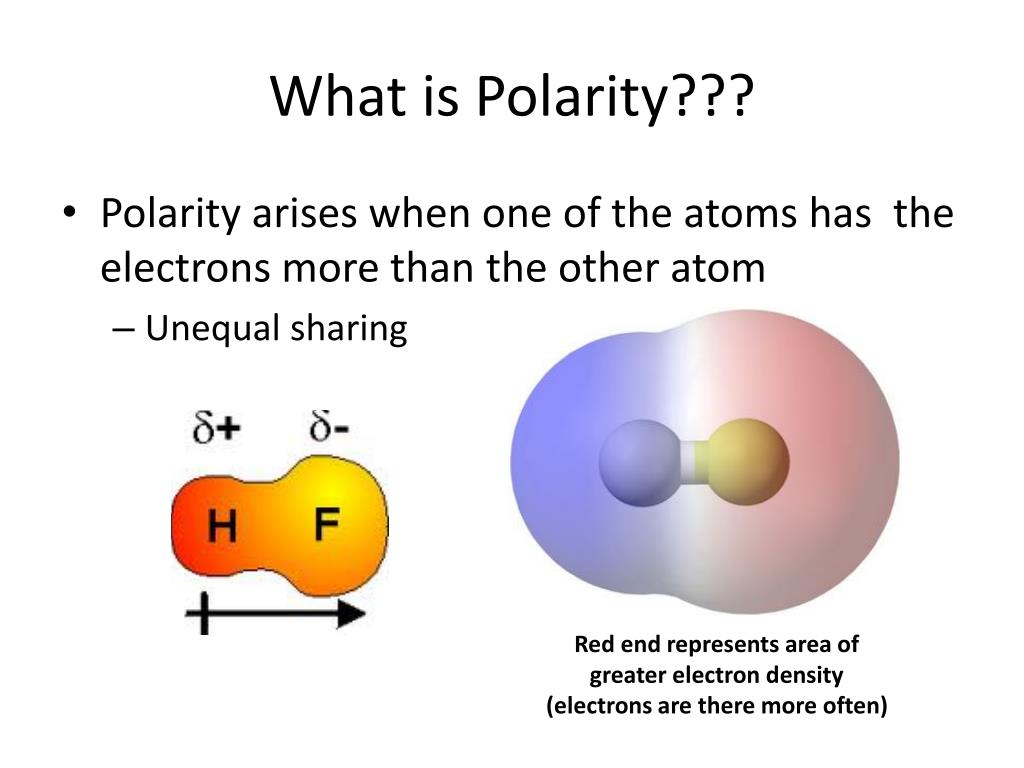
PPT Molecular Geometry Shapes! PowerPoint Presentation, free
Polarity Thinking 101 Intro to the Power of Polarities (+ Visuals) Sloww
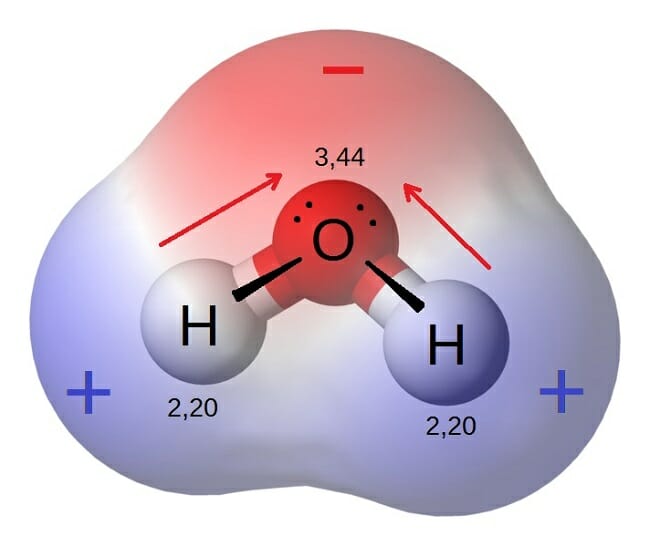
Polar Molecule Definition and Examples Biology Dictionary

Chapter 5.6 Properties of Polar Covalent Bonds Chemistry LibreTexts
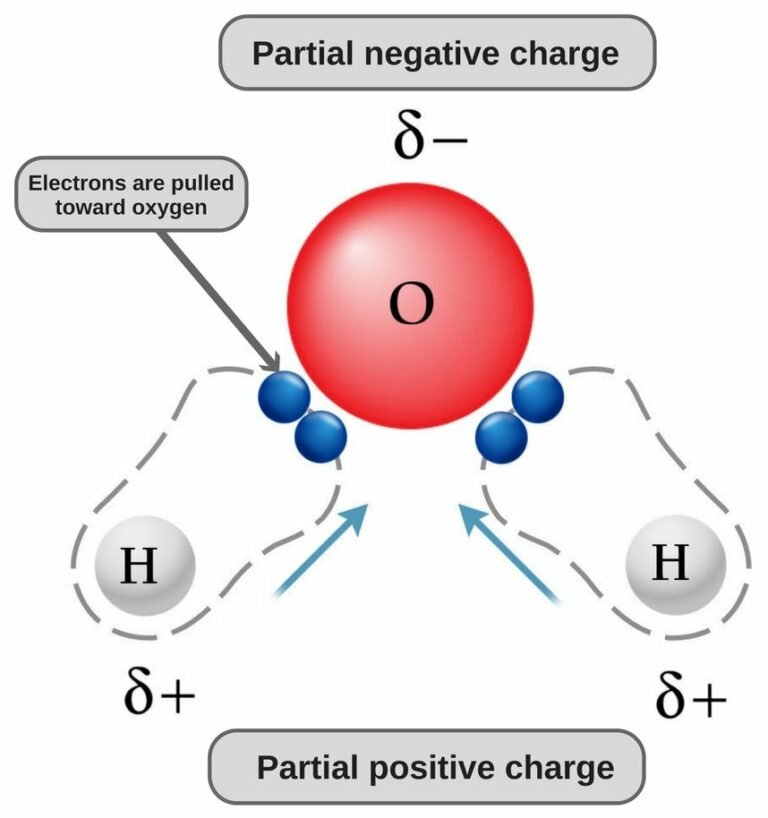
H2O Polar or Nonpolar Check Covalent bond and polarity Geometry of
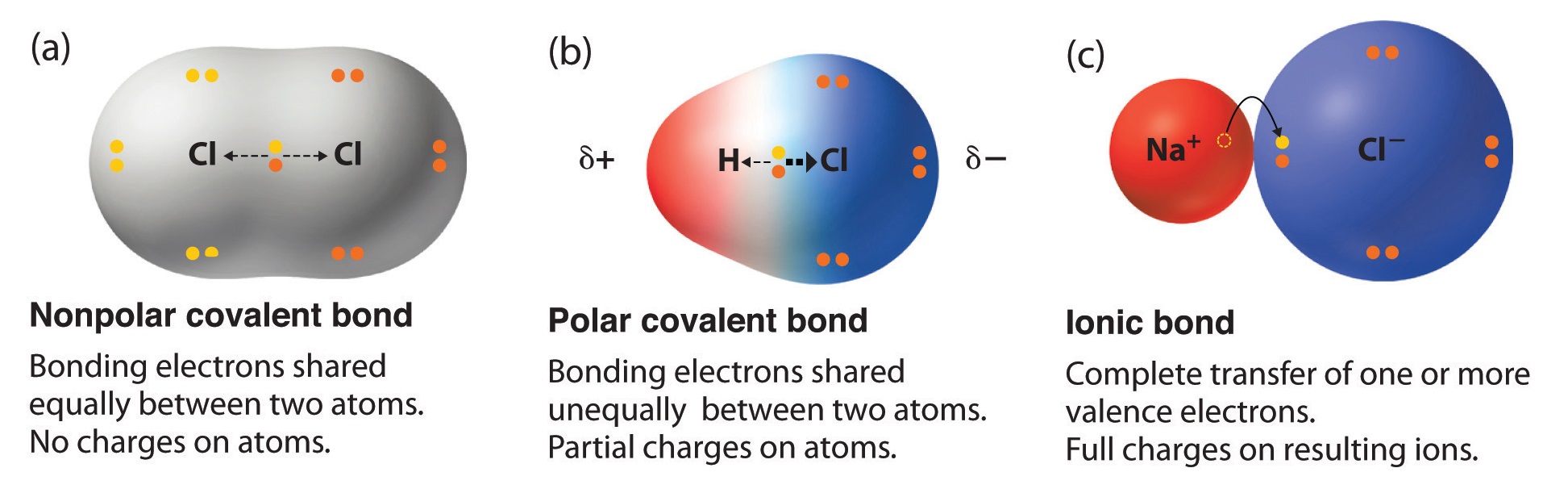
9.3 Molecular Shape and Molecular Polarity Chemistry LibreTexts
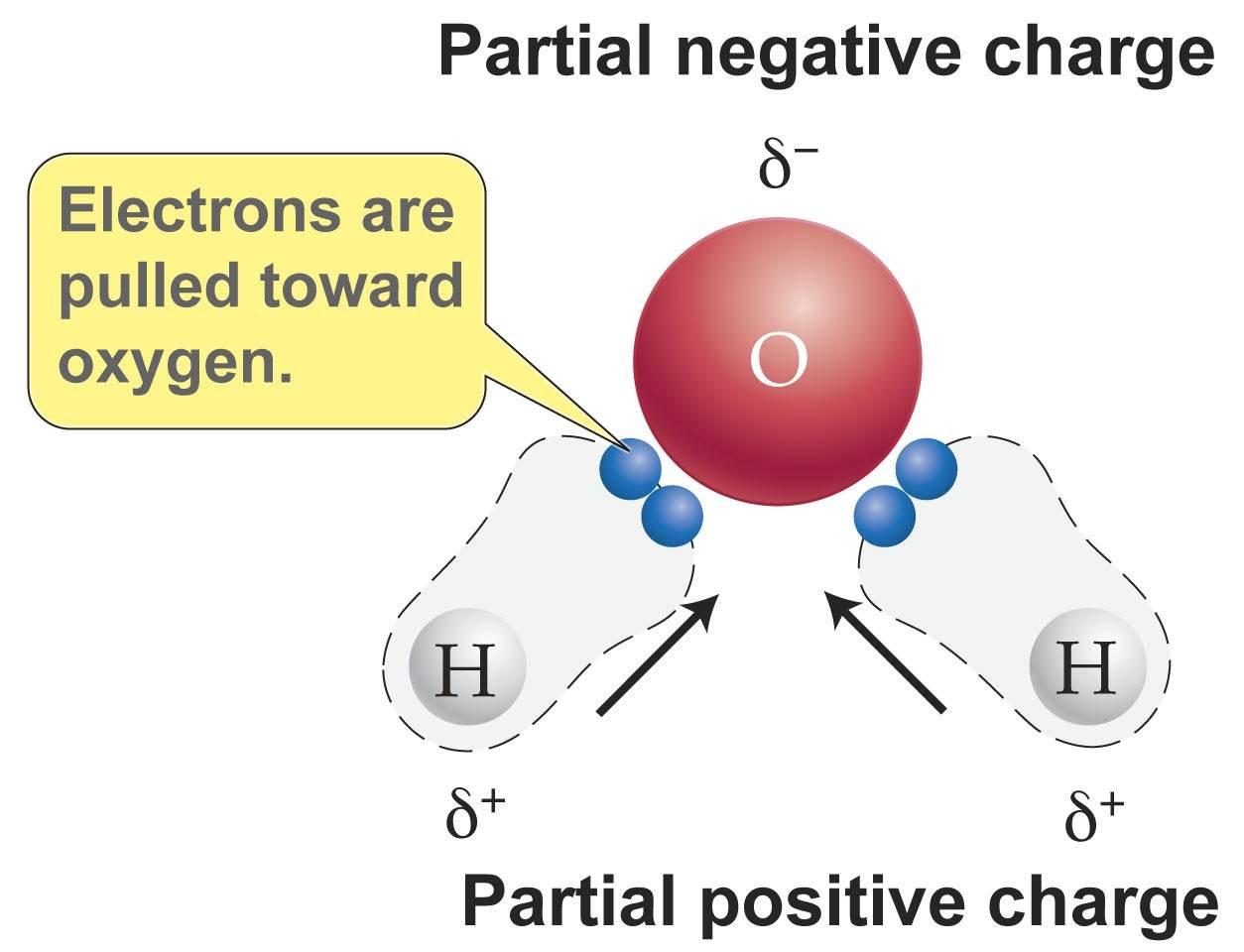
What kinds of molecules are polar? + Example
Explain How Polar Compounds Differ From Nonpolar Compounds.
There Is An Abundance Of Experimental Evidence To That Effect—From Their Physical.
Predict The Structures Of Small Molecules Using Valence Shell Electron Pair Repulsion (Vsepr) Theory.
It Explains How To Indicate The Polarity Of A Bond And Of A.
Related Post: