Molecular Geometry Drawing
Molecular Geometry Drawing - A draw the lewis electron structure of the molecule or polyatomic ion. Explore molecule shapes by building molecules in 3d! Then count the charge clouds to determine electron pair geometry. If there are no lone pairs, the molecular geometry is the same as the electron pair geometry. A table of geometries using the vsepr theory can facilitate drawing and understanding molecules. The molecule has three atoms in a plane in equatorial positions and two atoms above and below the plane in axial positions. The structural formula editor is surround by three toolbars which contain the tools you can use in the editor. In this video we’ll use vspre theory to practice the rules for identifying the major molecular geometries, including. Web molecular geometries (linear, trigonal, tetrahedral, trigonal bipyramidal, and octahedral) are determined by the vsepr theory. Web the molecular geometry of pcl 5 is trigonal bipyramidal, as shown in figure \(\pageindex{3}\). Then, compare the model to real molecules! Compare your models with real molecules. Web the molecular geometry of pcl 5 is trigonal bipyramidal, as shown in figure \(\pageindex{3}\). Explore molecule shapes by building molecules in 3d! A draw the lewis electron structure of the molecule or polyatomic ion. In this video we’ll use vspre theory to practice the rules for identifying the major molecular geometries, including. Explore molecule shapes by building molecules in 3d! Then count the charge clouds to determine electron pair geometry. The second figure serves as a visual aid for the table. If there are one or more lone pairs (or single electron), the molecular. Find out by adding single, double or triple bonds and lone pairs to the central atom. Web we recommend using the latest version of chrome, firefox, safari, or edge. Explore molecule shapes by building molecules in 3d! Web build and explore molecules in 3d with this interactive simulation. The molecule has three atoms in a plane in equatorial positions and. Web to predict electron pair geometry and molecular geometry, first draw the lewis structure. 370k views 6 years ago. Explore molecule shapes by building molecules in 3d! Compare your models with real molecules. Learn how molecule shapes change with different bonds and electron pairs. Understanding the molecular structure of a compound can help determine the polarity, reactivity, phase of matter, color, magnetism, as well as the biological activity. Web molecular geometries (linear, trigonal, tetrahedral, trigonal bipyramidal, and octahedral) are determined by the vsepr theory. A table of geometries using the vsepr theory can facilitate drawing and understanding molecules. A draw the lewis electron structure. Web to predict electron pair geometry and molecular geometry, first draw the lewis structure. Web build and explore molecules in 3d with this interactive simulation. How does molecule shape change with different numbers of bonds and electron pairs? Web using the vsepr model, predict the molecular geometry of each molecule or ion. In this video we’ll use vspre theory to. Molview consists of two main parts, a structural formula editor and a 3d model viewer. Web using the vsepr model, predict the molecular geometry of each molecule or ion. Learn how molecule shapes change with different bonds and electron pairs. Pf 5 (phosphorus pentafluoride, a catalyst used in certain organic reactions) h 3 0 + (hydronium ion) given: Then, compare. Find out by adding single, double or triple bonds and lone pairs to the central atom. If there are one or more lone pairs (or single electron), the molecular geometry is different. Understanding the molecular structure of a compound can help determine the polarity, reactivity, phase of matter, color, magnetism, as well as the biological activity. Compare your models with. The structural formula editor is surround by three toolbars which contain the tools you can use in the editor. Web the molecular geometry of pcl 5 is trigonal bipyramidal, as shown in figure \(\pageindex{3}\). 370k views 6 years ago. The second figure serves as a visual aid for the table. The molecule has three atoms in a plane in equatorial. Explore molecule shapes by building molecules in 3d! Learn how molecule shapes change with different bonds and electron pairs. Web we recommend using the latest version of chrome, firefox, safari, or edge. Find out by adding single, double or triple bonds and lone pairs to the central atom. Web to predict electron pair geometry and molecular geometry, first draw the. Find out by adding single, double or triple bonds and lone pairs to the central atom. Web we recommend using the latest version of chrome, firefox, safari, or edge. Web molecular geometries (linear, trigonal, tetrahedral, trigonal bipyramidal, and octahedral) are determined by the vsepr theory. Pf 5 (phosphorus pentafluoride, a catalyst used in certain organic reactions) h 3 0 + (hydronium ion) given: If there are no lone pairs, the molecular geometry is the same as the electron pair geometry. 370k views 6 years ago. Web the molecular geometry of pcl 5 is trigonal bipyramidal, as shown in figure \(\pageindex{3}\). Then count the charge clouds to determine electron pair geometry. A table of geometries using the vsepr theory can facilitate drawing and understanding molecules. How does molecule shape change with different numbers of bonds and electron pairs? Once you’ve drawn a molecule, you can click the 2d to 3d button to convert the molecule into a 3d model which is then. The structural formula editor is surround by three toolbars which contain the tools you can use in the editor. If there are one or more lone pairs (or single electron), the molecular geometry is different. Explore molecule shapes by building molecules in 3d! Learn how molecule shapes change with different bonds and electron pairs. Then, compare the model to real molecules!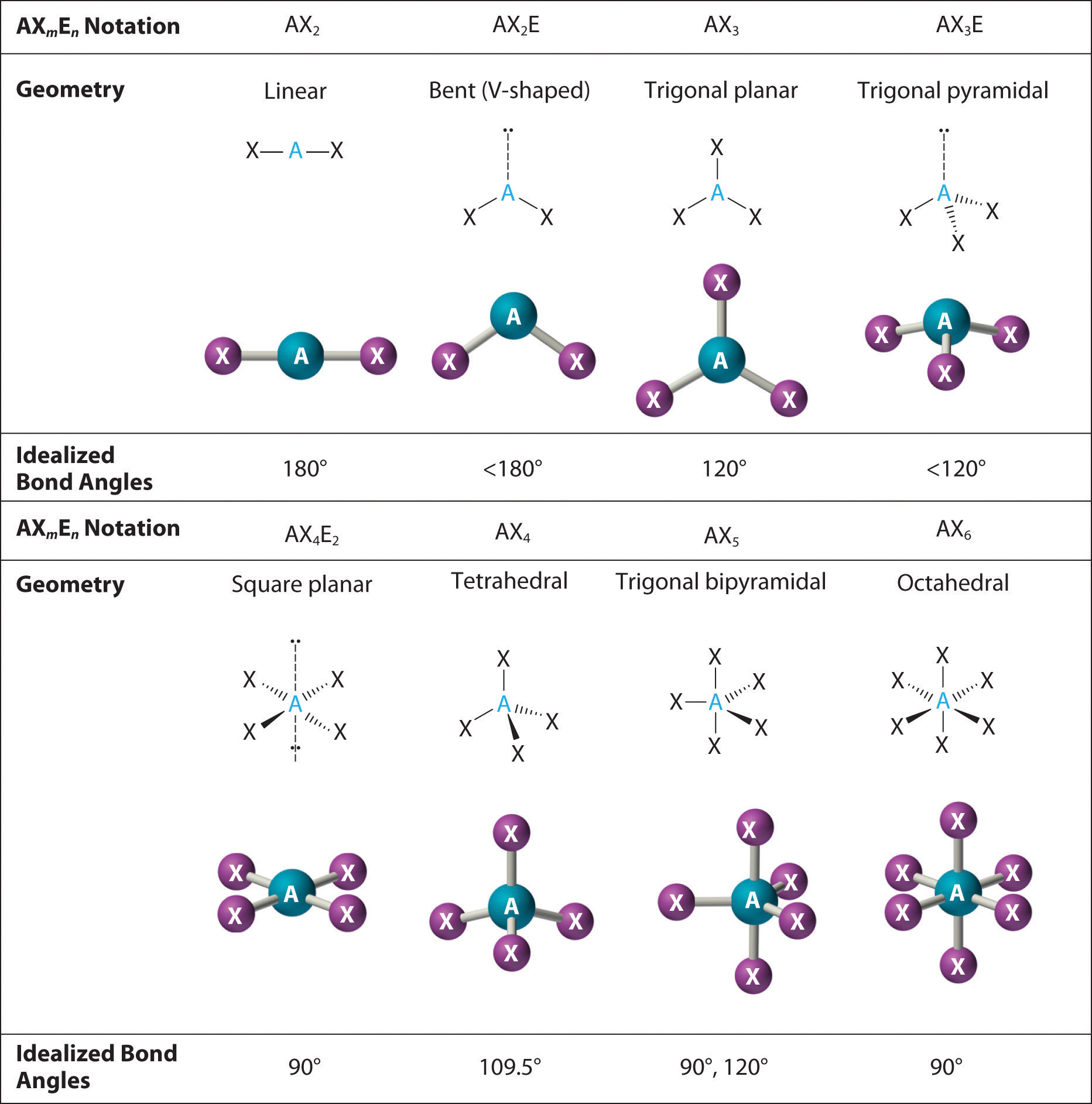
9.7 The Shapes of Molecules Chemistry LibreTexts

Molecular Geometry Boundless Chemistry
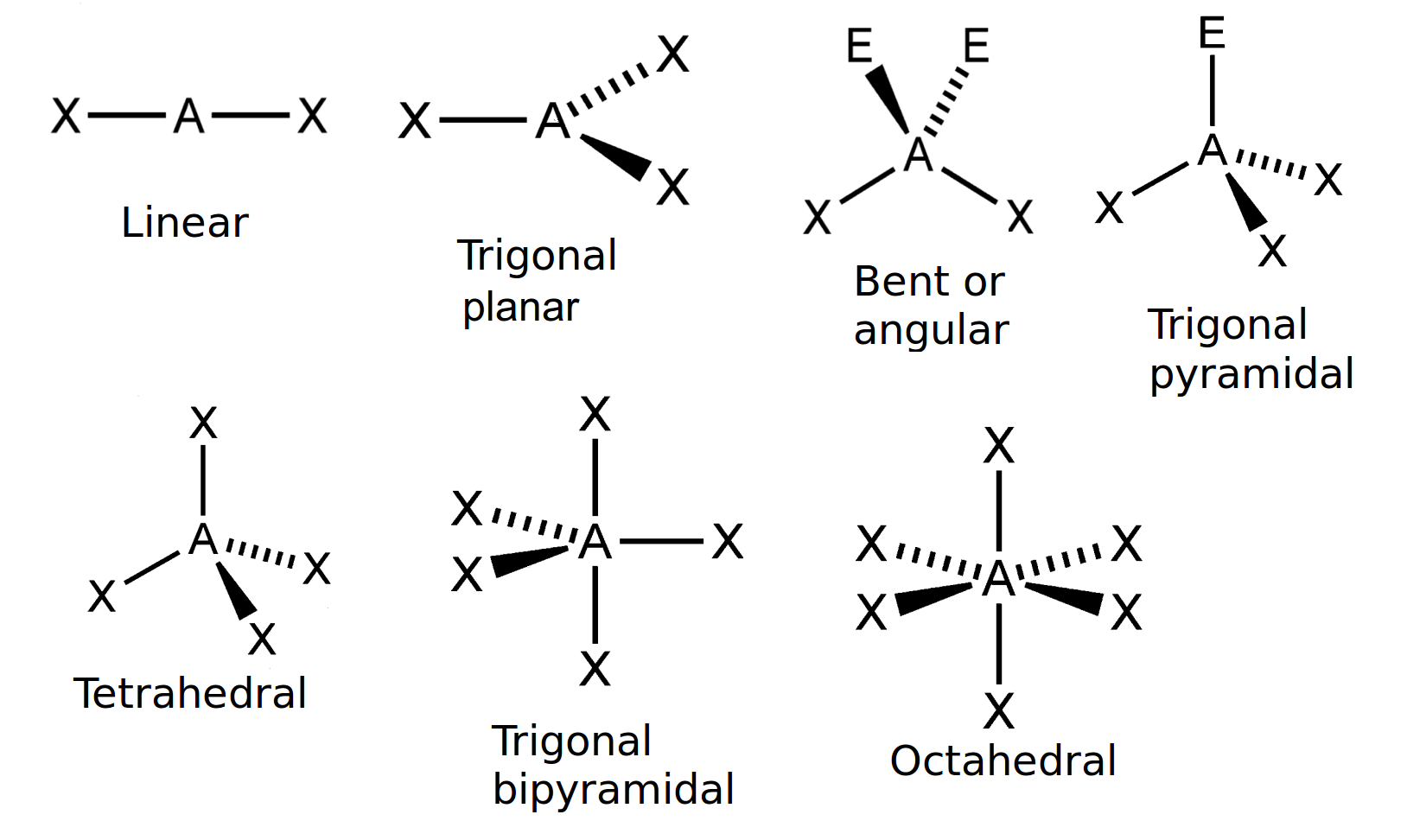
3.2 Molecular shape Atomic combinations Siyavula
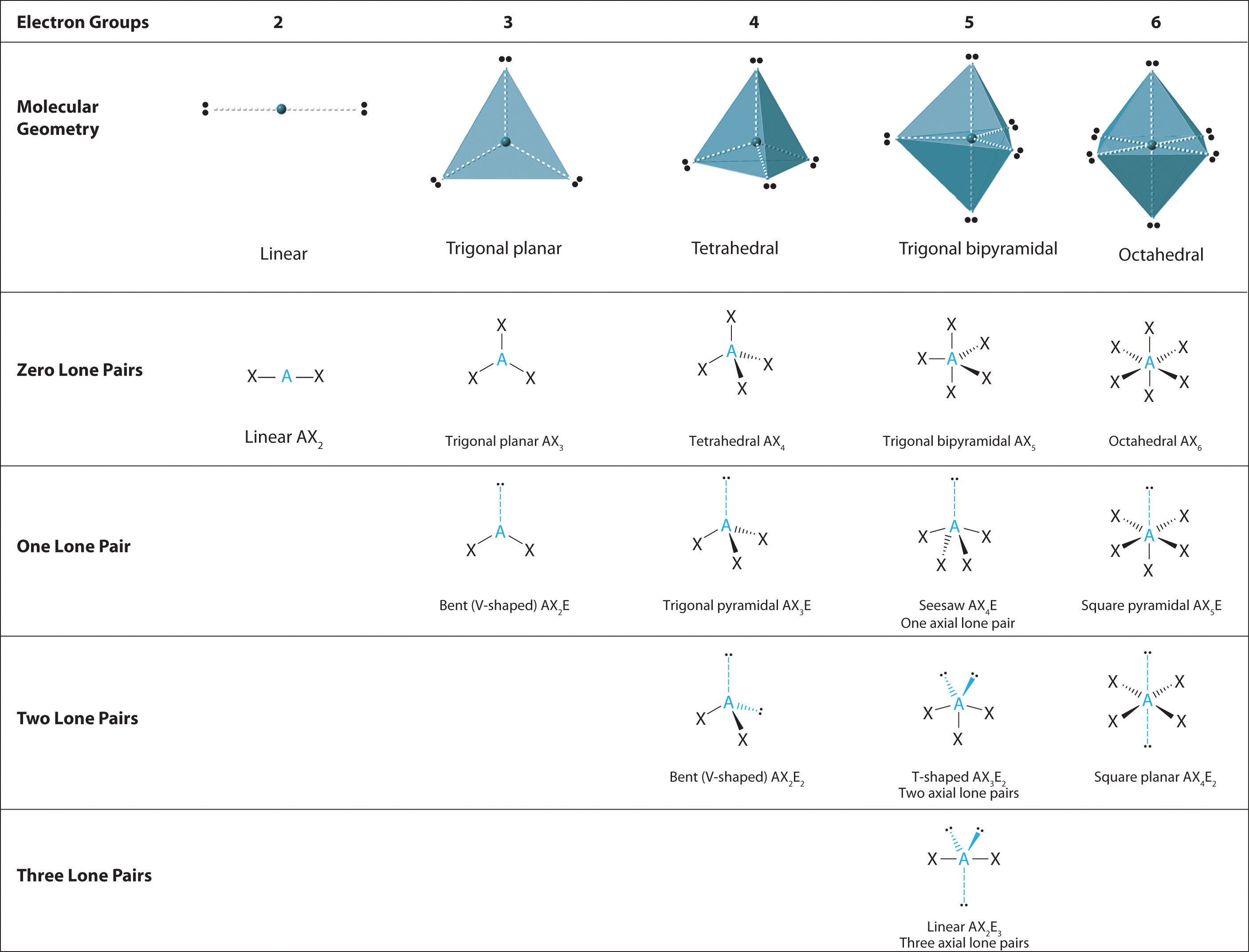
9.7 The Shapes of Molecules Chemistry LibreTexts

H2O Lewis Structure, Molecular Geometry, and Hybridization

CF4 Lewis Structure, Molecular Geometry, Hybridization, and Polarity

2.6 Molecular Structure and Polarity Chemistry for Chemical

C2H2 Molecular Geometry / Shape and Bond Angles (see description for
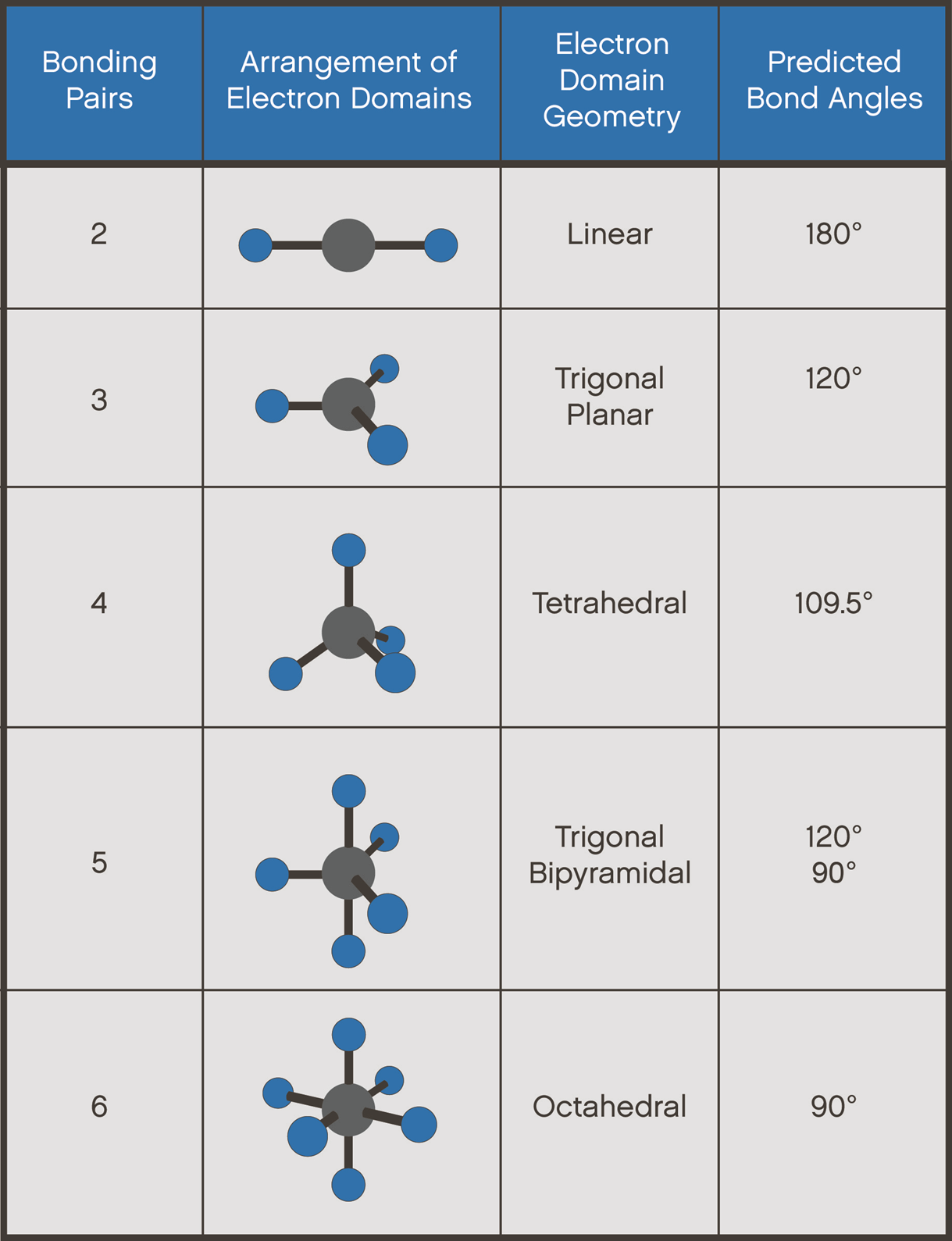
Diagram of shapes of molecules, showng bonding pairs, arrangement of
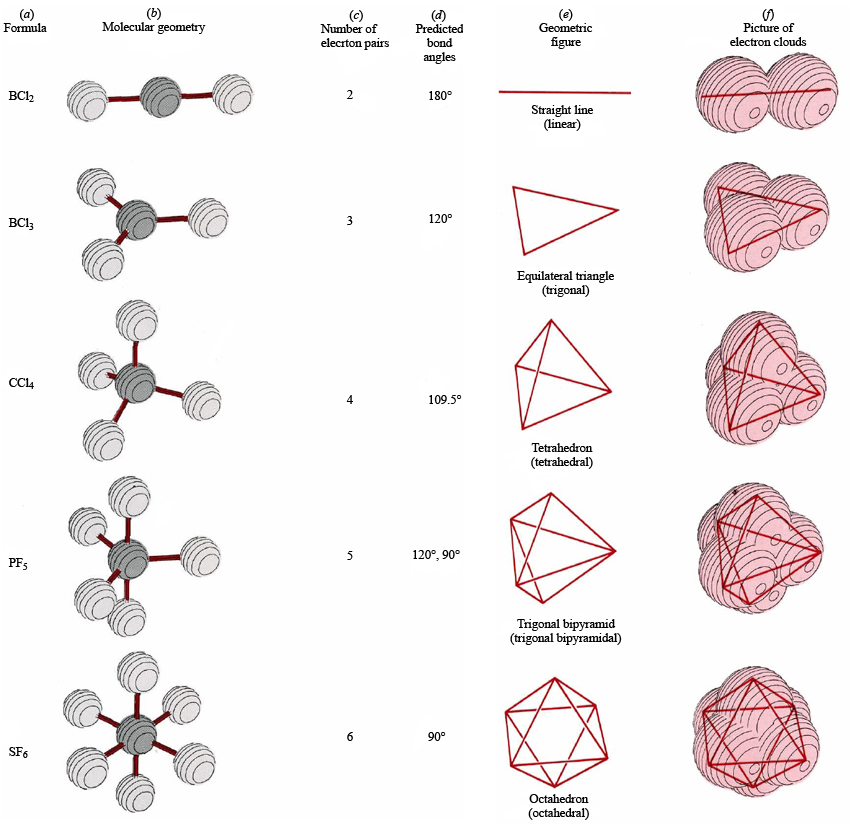
Molecular Geometry Chemistry Socratic
Compare Your Models With Real Molecules.
In This Video We’ll Use Vspre Theory To Practice The Rules For Identifying The Major Molecular Geometries, Including.
Molview Consists Of Two Main Parts, A Structural Formula Editor And A 3D Model Viewer.
Understanding The Molecular Structure Of A Compound Can Help Determine The Polarity, Reactivity, Phase Of Matter, Color, Magnetism, As Well As The Biological Activity.
Related Post: