Ionic Bonding Drawing
Ionic Bonding Drawing - The astute reader may have noticed something: Swap the crosses for dots in one of your diagrams. 292k views 3 years ago new ap & general chemistry video playlist. Web one line is a single bond with 2 bonding electrons, two lines is a double bond with 4 bonding electrons, and three lines is a triple bond with 6 bonding electrons. Compounds can be classified as ionic or covalent. Web i want to help you achieve the grades you (and i) know you are capable of; During ionic bonding the atoms form ions by magnesium has two electrons in its outer gaining or losing electrons to obtain a full outer shell, oxygen has six. State the limitations of a range of models used to represent ionic bonding. Covalent bonds form when two atoms react such that they share electrons in a bond between them and each atom donates half of the electrons which forms the bond from their original. Look at the number of electrons on the outer shell of each atom. Explain how octet stability is satisfied by electron sharing and electron transfer. Web © 2023 google llc. Web i want to help you achieve the grades you (and i) know you are capable of; Web one line is a single bond with 2 bonding electrons, two lines is a double bond with 4 bonding electrons, and three lines is a. Look at the number of electrons on the outer shell of each atom. Web i want to help you achieve the grades you (and i) know you are capable of; Web draw the electron configuration diagram for each atom. The astute reader may have noticed something: Electron configuration diagrams (see rsc.li/2whsi4f if you need a reminder). Sodium chloride crystals are brittle. Magnesium has two electrons in its outer shell, oxygen has six. Draw the electron configuration diagram for each atom. These oppositely charged ions attract each other to form ionic networks (or lattices). Web define ionic bond. For example, sodium cations (positively charged ions) and chlorine anions (negatively charged ions) are connected via ionic bonds in sodium chloride, or table salt. Web i want to help you achieve the grades you (and i) know you are capable of; State the limitations of a range of models used to represent ionic bonding. During ionic bonding the atoms form. It is a type of chemical bond that generates two oppositely charged ions. Web revision notes on 1.6.4 ionic bonds: Define electronegativity and dipole moment. Web draw dot and cross diagrams of ionic bonding and explain how an ionic lattice is held together. Molecules are the simplest unit of a covalent compound, and molecules can be represented in many different. Sodium chloride crystals are brittle. Opposite charges attract and like charges repel. Molecules are the simplest unit of a covalent compound, and molecules can be represented in many different ways. Many of the ions that form have eight electrons in their valence shell. Sodium chloride has a high melting and boiling point. While you are learning how to draw dot and cross diagrams it’s useful to start with something you are already familiar with: Look at the number of electrons on the outer shell of each atom. Web sketch the lewis structure for a given compound. Web © 2023 google llc. Web i want to help you achieve the grades you (and. Ionic bonding occurs when atoms lose and gain electrons to form ions and then the positively and negatively charged ions are attracted to each other. 6.7k views 7 years ago edexcel chemistry 2022: In section 4.7, we demonstrated that ions are formed by losing electrons to make cations, or by gaining electrons to form anions. Ionic bonding is the complete. Web sketch the lewis structure for a given compound. 224k views 5 years ago. Swap the crosses for dots in one of your diagrams. Web i want to help you achieve the grades you (and i) know you are capable of; Ionic bonding is the complete transfer of valence electron (s) between atoms. Web i want to help you achieve the grades you (and i) know you are capable of; Atoms are the smallest units of matter that still retain the fundamental chemical properties of an element. 1.21b explain how to draw ionic bonding.more. Draw the electron configuration diagrams for each atom in the compound. When drawing lewis dot structures for ionic compounds. Web © 2023 google llc. Covalent bonds form when two atoms react such that they share electrons in a bond between them and each atom donates half of the electrons which forms the bond from their original. Compounds can be classified as ionic or covalent. Ionic bonding occurs when atoms lose and gain electrons to form ions and then the positively and negatively charged ions are attracted to each other. Ionic bonding is the complete transfer of valence electron (s) between atoms. Lewis diagram of the cyanide ion (cn⁻) exceptions to the octet rule. Understand how electron states can be mixed to form hybrid orbitals. Draw the electron configuration diagram for each atom. During ionic bonding the atoms form ions by gaining or losing electrons to obtain a full outer shell. Web one line is a single bond with 2 bonding electrons, two lines is a double bond with 4 bonding electrons, and three lines is a triple bond with 6 bonding electrons. State the limitations of a range of models used to represent ionic bonding. Web draw the electron configuration diagram for each atom. While you are learning how to draw dot and cross diagrams it’s useful to start with something you are already familiar with: These grades are the stepping stone to your future. For example, sodium cations (positively charged ions) and chlorine anions (negatively charged ions) are connected via ionic bonds in sodium chloride, or table salt. Electrostatics explains why this happens: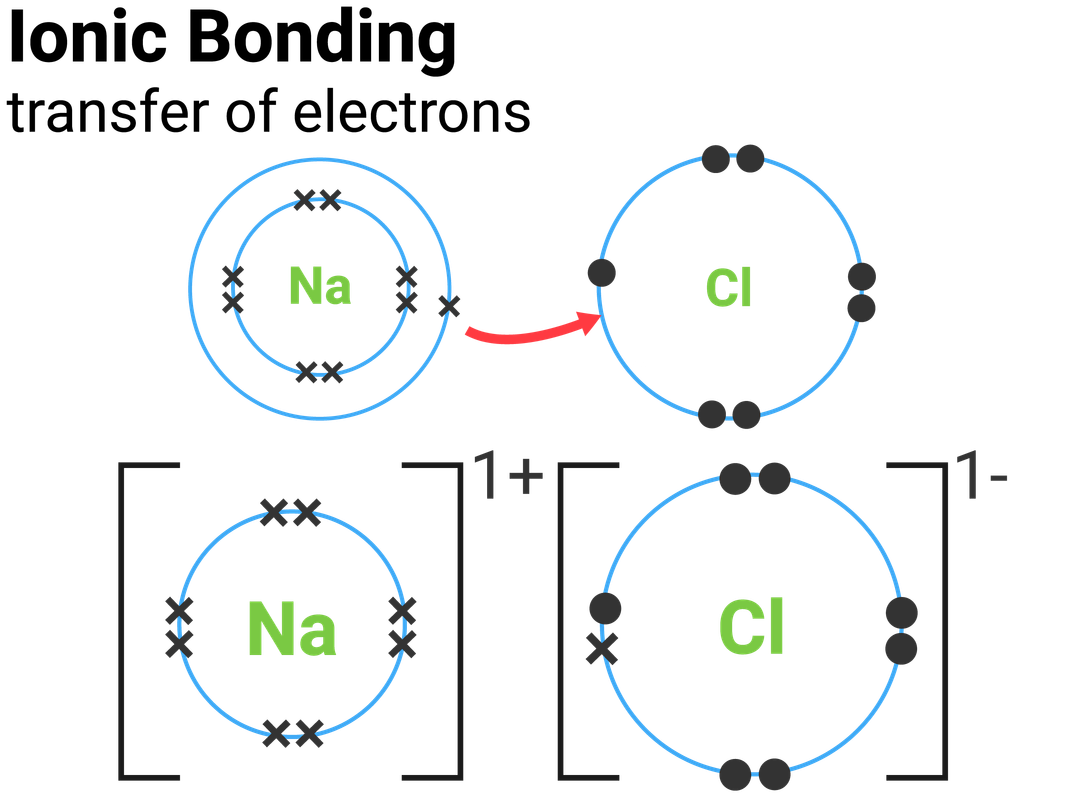
6 ionic bonding 01 — Postimages

Bonding and Properties of materials OCR Gateway C2 revisechemistry.uk
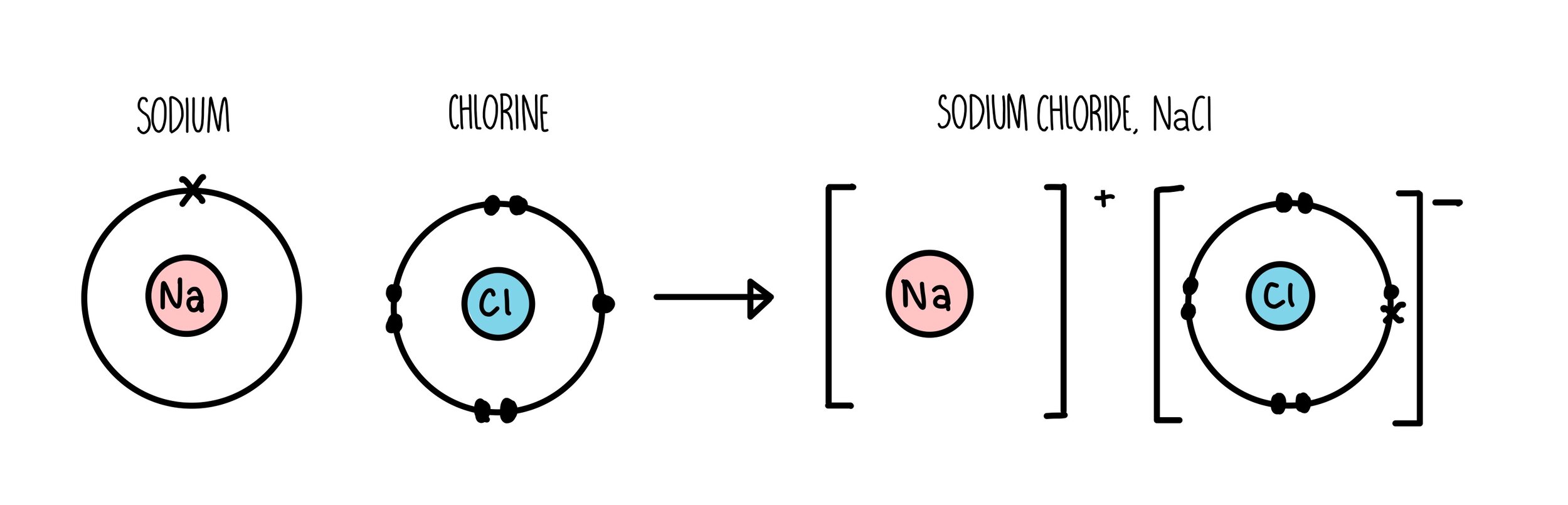
Ionic Bonding — the science hive
Examples of Ionic Bonds and Compounds

FileIonic bonding.svg Wikimedia Commons
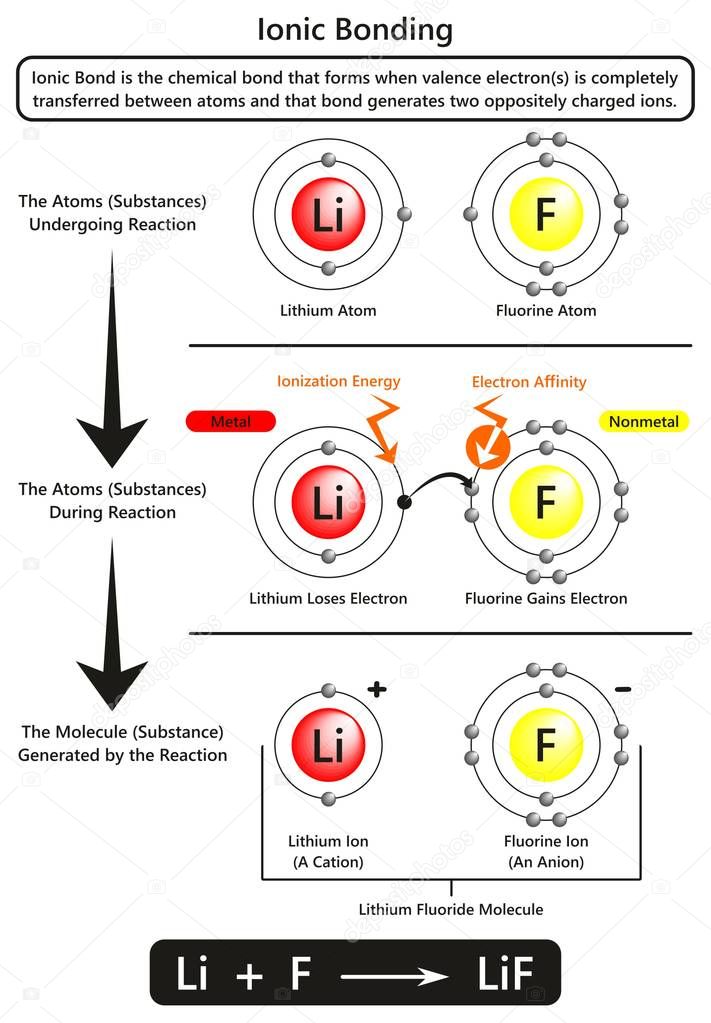
Ionic bonding infographic diagram with example of Ionic bond between

Ionic bonding Wikipedia
:max_bytes(150000):strip_icc()/ionic-bond-58fd4ea73df78ca1590682ad.jpg)
Examples of Ionic Bonds and Compounds

ionic bond Definition, Properties, Examples, & Facts Britannica

chemical bonding Ionic and covalent compounds Britannica
When Drawing Lewis Dot Structures For Ionic Compounds You Need To Follow A Different Set Of Rules Than With Lewis Structures For Covalent/Molecular Compounds.
Swap The Crosses For Dots In One Of Your Diagrams.
Web I Want To Help You Achieve The Grades You (And I) Know You Are Capable Of;
The Astute Reader May Have Noticed Something:
Related Post: