How To Draw Resonance Structures
How To Draw Resonance Structures - All resonance structures must be valid lewis structures. Web draw the resonance structures of molecules or ions that exhibit delocalization. So let's draw the resonance structure. Let's make the ones on the top left here red. Also, you will learn how to dr. The electrons move over to here, to here and then finally, to here. It explains how to identify the major resonance contributor as well as the minor. Determine the relative stability of resonance structures using a set of rules. In resonance structures, it does not require to show transformation of electrons by arrows. Web guidelines for drawing resonance structures: Web guidelines for drawing resonance structures: Curved arrows communicate electron flow (movement) In following examples, arrows are used to show electrons transformation. Use the concept of resonance to explain structural features of molecules and ions. Resonance is a way of describing delocalized electrons within certain molecules or polyatomic ions where the bonding cannot be expressed by a single lewis formula. How many resonance structures does the resultant anion have? Let's make the ones on the top left here red. Web we draw our resonance brackets and go ahead and draw our other resonance structure for benzene. Web when learning to draw and interpret resonance structures, there are a few basic guidelines to help. Some molecules have two or more chemically. This is the acetate anion, and this dot structure does not completely describe the acetate anion; It explains how to draw the resonance structures using curved. But, to identify each resonance structures, it is good to show arrows. Web in this video, you'll become an expert at identifying and drawing resonance structures for organic molecules. Definition, examples, and how to. Web how to draw resonance structures. All resonance structures must be valid lewis structures. This is the acetate anion, and this dot structure does not completely describe the acetate anion; ( keep in mind that all the rules applied to lewis structures still apply here!) all resonance structures must have the same atom connectivity and only differ in the electron. Oxygen atoms (3*6) = 18. This is the acetate anion, and this dot structure does not completely describe the acetate anion; Guidelines for drawing resonance structures: In resonance structures, it does not require to show transformation of electrons by arrows. Web in this video, you'll become an expert at identifying and drawing resonance structures for organic molecules. How many resonance structures does the resultant anion have? Web this general chemistry video tutorial provides a basic introduction into resonance structures. So let's draw the resonance structure. In chemistry, resonance, also called mesomerism, is a way of describing bonding in certain molecules or polyatomic ions by the combination of several contributing structures into a resonance hybrid in valence bond. How many resonance structures does the resultant anion have? Oxygen atoms (3*6) = 18. It explains how to draw the resonance structures using curved. Calculate the total number of valence electrons from each atom. In following examples, arrows are used to show electrons transformation. Use the concept of resonance to explain structural features of molecules and ions. Resonance is a way of describing delocalized electrons within certain molecules or polyatomic ions where the bonding cannot be expressed by a single lewis formula. Some molecules have two or more chemically equivalent lewis electron structures, called resonance structures. Web this lecture is about resonance structures in. Determine the relative stability of resonance structures using a set of rules. Web draw the resonance structures of molecules or ions that exhibit delocalization. Web to draw all resonance structures, take the lewis structure we drawn by using vespr rule. Some molecules have two or more chemically equivalent lewis electron structures, called resonance structures. Web this tutorial has several examples. Resonance is a mental exercise and method within the valence bond theory of bonding that describes the delocalization of electrons within molecules. And so, let's follow those electrons. In resonance structures, it does not require to show transformation of electrons by arrows. Calculate the total number of valence electrons from each atom. Sometimes one dot structures is not enough to. Web resonance is a way of describing delocalized electrons within certain molecules or polyatomic ions where the bonding cannot be expressed by a single lewis formula. All resonance structures must be valid lewis structures. The electrons move over to here, to here and then finally, to here. Home / physical chemistry / resonance structures. Calculate the total number of valence electrons from each atom. Web this organic chemistry video tutorial provides a basic introduction into drawing resonance structures. Web how to draw resonance structures. So let's draw the resonance structure. But, to identify each resonance structures, it is good to show arrows. Web to draw all resonance structures, take the lewis structure we drawn by using vespr rule. And so, let's follow those electrons. Web this tutorial has several examples of drawing resonance structures as well as explains in more detail the concept of resonance. Some molecules have two or more chemically equivalent lewis electron structures, called resonance structures. In resonance structures, it does not require to show transformation of electrons by arrows. Web this general chemistry video tutorial provides a basic introduction into resonance structures. How many resonance structures does the resultant anion have?
1.3 Resonance Structures Organic Chemistry I

how to draw resonance structure YouTube
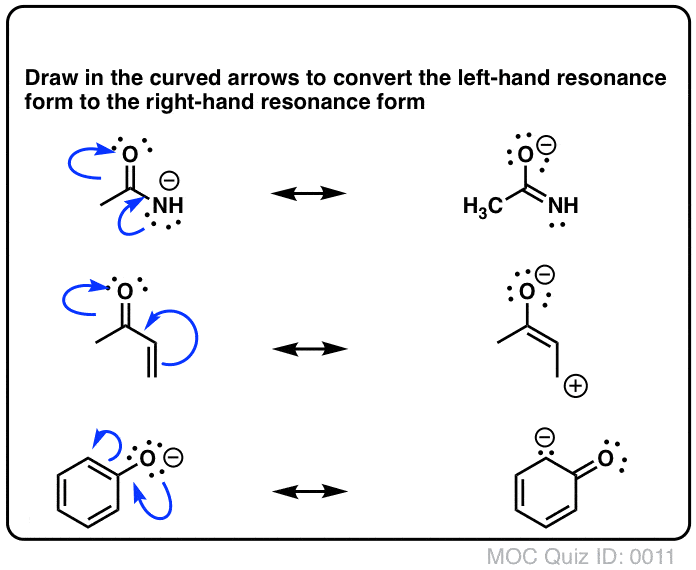
Resonance Structures Practice Master Organic Chemistry

How to draw resonance structures YouTube

How To Draw Resonance Structures YouTube

Resonance Structures, Basic Introduction How To Draw The Resonance

How to Draw Resonance Contributors MCC Organic Chemistry

Resonance Structures Easy Hard Science
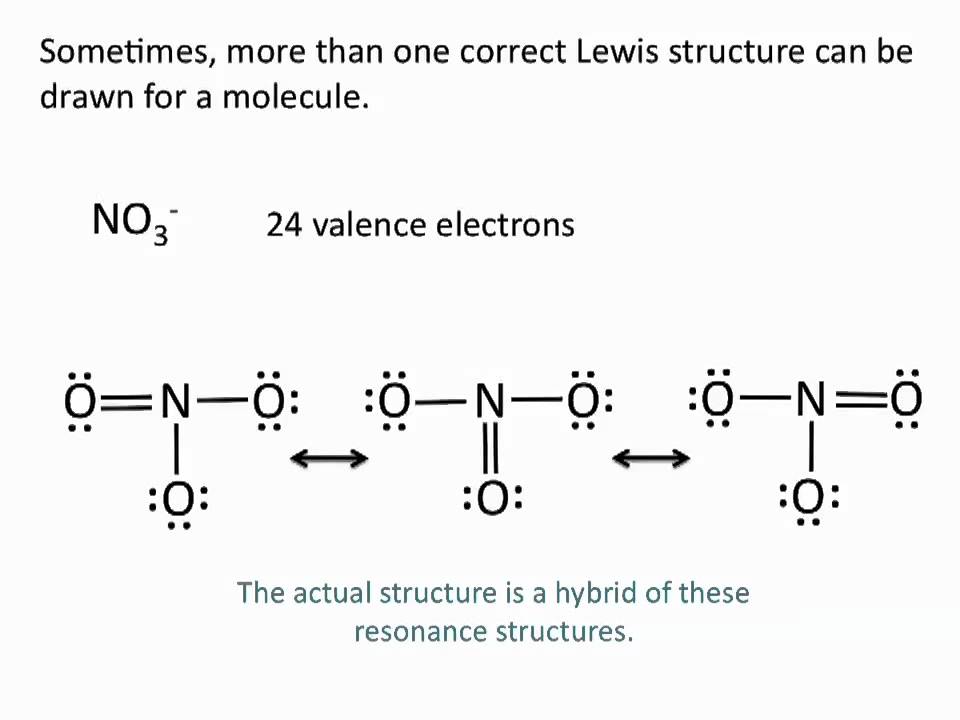
Drawing Lewis Structures Resonance Structures Chemistry Tutorial
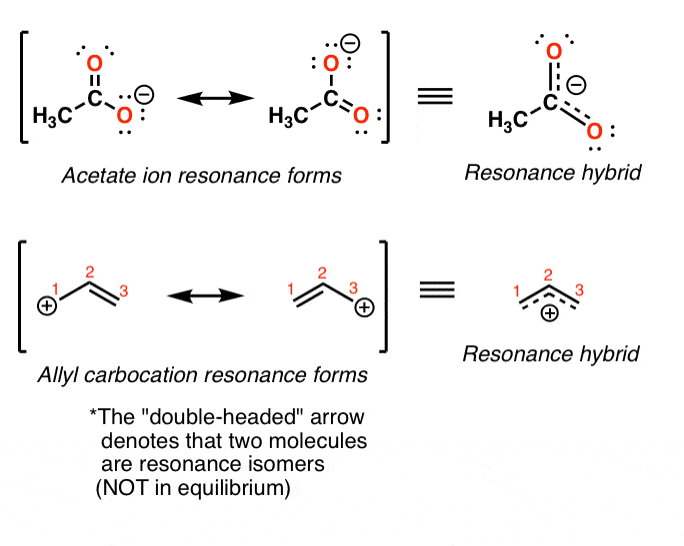
Resonance Structures 4 Rules On How To Evaluate Them, With Practice
Let's Make The Ones On The Top Left Here Red.
Guidelines For Drawing Resonance Structures:
Use The Concept Of Resonance To Explain Structural Features Of Molecules And Ions.
Use The Concept Of Resonance To Explain Structural Features Of Molecules And Ions.
Related Post: