How To Draw Resonance Structures Organic Chemistry
How To Draw Resonance Structures Organic Chemistry - Web in this video, you'll become an expert at identifying and drawing resonance structures for organic molecules. Web the discussion of resonance effect heavily relies on the understanding of resonance structures. 3 common mistakes to avoid; Web as we’ve seen in previous posts, four key factors that determine the importance of resonance structures in organic chemistry are: Sometimes one dot structures is not enough to completely describe a molecule or an ion, sometimes you need two or more, and here's an example: Chemistry · carbohydrates · psychology · institutional How to find the best resonance structure by applying electronegativity; Web here, we will focus on how to draw resonance structures (or resonance contributors) for organic chemistry species and how to compare the relative stabilities between the structures. Learn for free about math, art, computer programming, economics, physics, chemistry, biology, medicine, finance, history, and more. Web how to avoid common mistakes when drawing resonance structures. Web the discussion of resonance effect heavily relies on the understanding of resonance structures. Chemistry · carbohydrates · psychology · institutional Draw the lewis structure & resonance for the molecule (using solid lines for bonds). How to find the best resonance structure by applying electronegativity; How stable are the negative charges? Web as we’ve seen in previous posts, four key factors that determine the importance of resonance structures in organic chemistry are: Web how to avoid common mistakes when drawing resonance structures. 167k views 8 years ago #youcanlearnanything. When learning to draw and interpret resonance structures, there are a few basic guidelines to help. Web identify the incorrect resonating structure for. Web drawing lewis structures: There are two simple answers to. Web it is useful to combine the resonance structures into a single structure called the resonance hybrid that describes the bonding of the molecule. It explains how to draw the resonance structures using curved. Where there can be a double or triple bond, draw a dotted. This is the acetate anion, and this dot structure does not completely describe the acetate anion; How to avoid common mistakes when drawing resonance structures. Chemistry · carbohydrates · psychology · institutional In both examples, the carbon ring has one atom that is. It explains how to draw the resonance structures using curved. Web in this video, you'll become an expert at identifying and drawing resonance structures for organic molecules. Web draw the resonance structures of molecules or ions that exhibit delocalization. Acids, bases & electron flow. 3 common mistakes to avoid; There are two simple answers to this question: According to the resonance effect, the greater the number of resonance contributors, the greater the resonance stabilization effect, and the more stable the. The general approach is described below: Web khan academy organic chemistry. Web in this video, you'll become an expert at identifying and drawing resonance structures for organic molecules. No enrolment fees1000s of free courses This is the acetate anion, and this dot structure does not completely describe the acetate anion; Where there can be a double or triple bond, draw a dotted. Sometimes one dot structures is not enough to completely describe a molecule or an ion, sometimes you need two or more, and here's an example: Web rules for drawing and working with. Begin by watching this video: This organic chemistry video tutorial provides a basic introduction into drawing resonance structures. Web resonance structures are a set of two or more lewis structures that collectively describe the electronic bonding of a single polyatomic species including fractional bonds and fractional charges. That lone pair is participating in resonance, which makes this nitrogen sp two. Web in this video, you'll become an expert at identifying and drawing resonance structures for organic molecules. How stable are the negative charges? Web identify the incorrect resonating structure for the given charged species. Determine the relative stability of resonance structures using a set of rules. No enrolment fees1000s of free courses Begin by watching this video: Use the concept of resonance to explain structural features of molecules and ions. Web as we’ve seen in previous posts, four key factors that determine the importance of resonance structures in organic chemistry are: 341k views 11 years ago. This organic chemistry video tutorial provides a basic introduction into drawing resonance structures. Web and so, here's a situation where drawing a resonance structure helps clue us into what's actually happening: We have learned that lewis structure is a straightforward representation of valence shell electrons in an atom, ion, or molecule. According to the resonance effect, the greater the number of resonance contributors, the greater the resonance stabilization effect, and the more stable the. Sometimes one dot structures is not enough to completely describe a molecule or an ion, sometimes you need two or more, and here's an example: Web this general chemistry video tutorial provides a basic introduction into resonance structures. Web how to avoid common mistakes when drawing resonance structures. Want to join the conversation? Web identify the incorrect resonating structure for the given charged species. The general approach is described below: This organic chemistry video tutorial provides a basic introduction into drawing resonance structures. Chemistry · carbohydrates · psychology · institutional It compares and contrasts two or more possible lewis structures that can represent a particular molecule. This is the acetate anion, and this dot structure does not completely describe the acetate anion; Use the concept of resonance to explain structural features of molecules and ions. Evaluating resonance structures with negative charges; Here we will focus on how to draw resonance structures (or resonance contributors) for organic chemistry species, and how to compare the relative stabilities between the structures.
Drawing Lewis Structures Resonance Structures Chemistry Tutorial
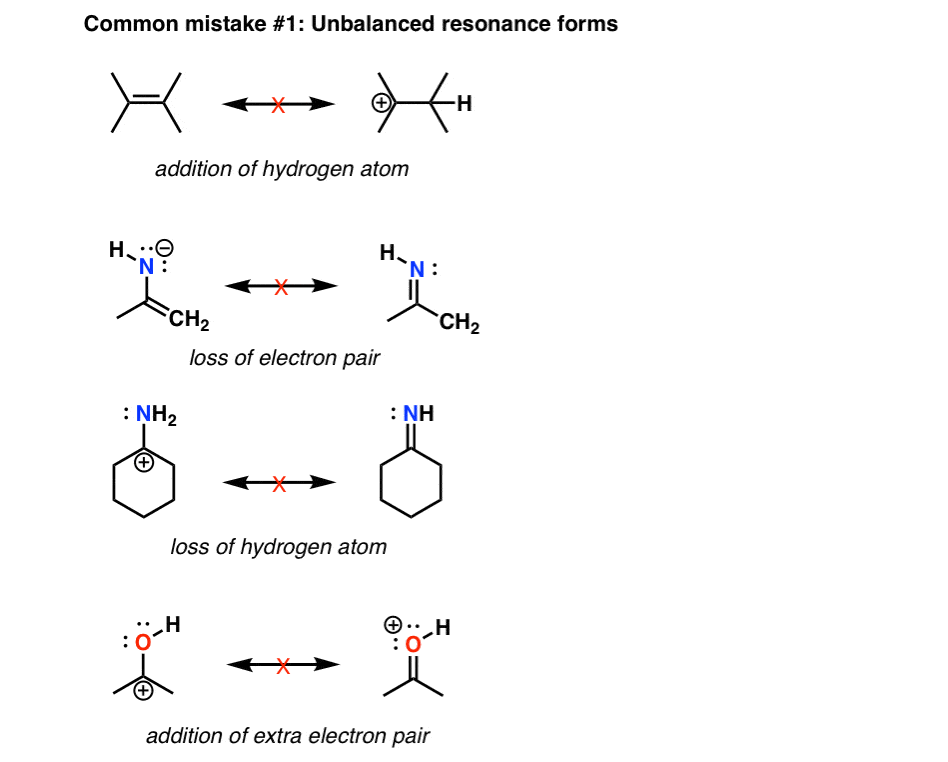
Drawing Resonance Structures 3 Common Mistakes To Avoid

Resonance Structures YouTube
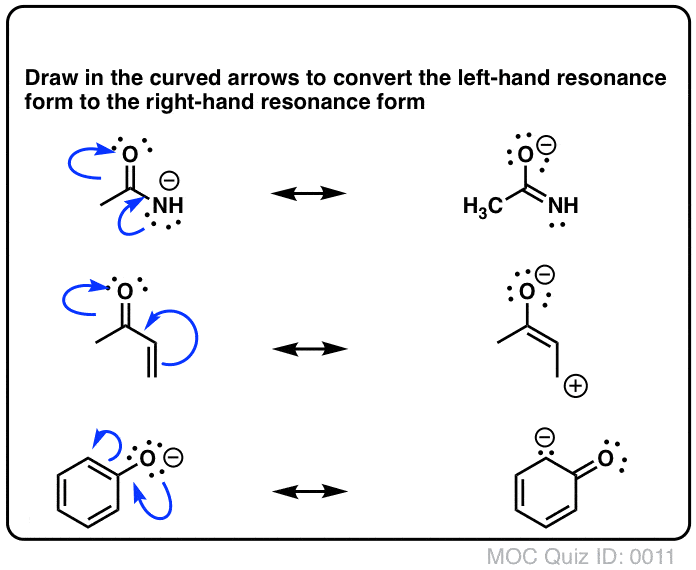
Resonance Structures Practice Master Organic Chemistry
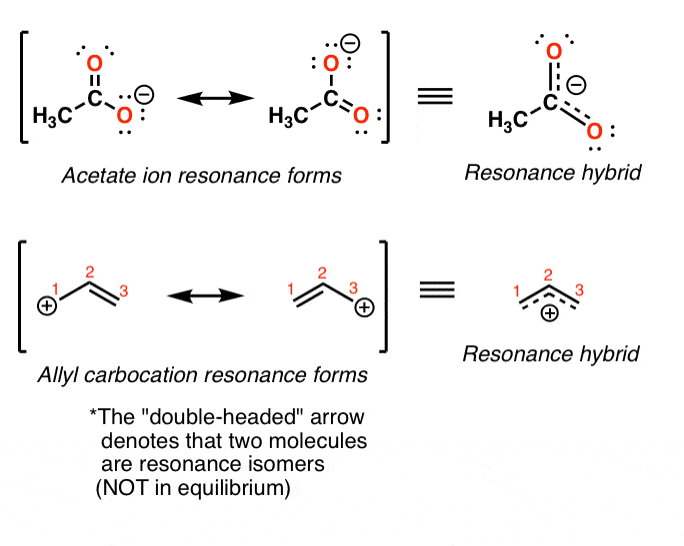
Resonance Structures 4 Rules On How To Evaluate Them, With Practice

How to Draw Resonance Contributors MCC Organic Chemistry
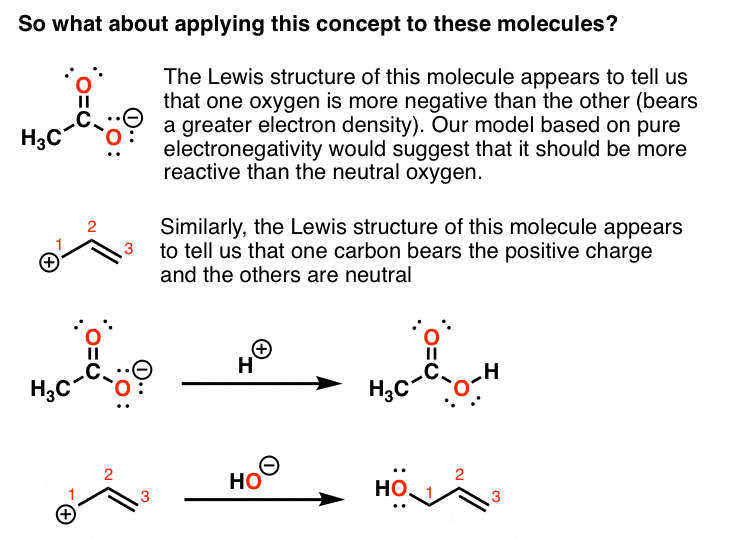
Introduction to resonance in organic chemistry — Master Organic Chemistry

6.2. Resonance Organic Chemistry 1 An open textbook

1.3 Resonance Structures Organic Chemistry I
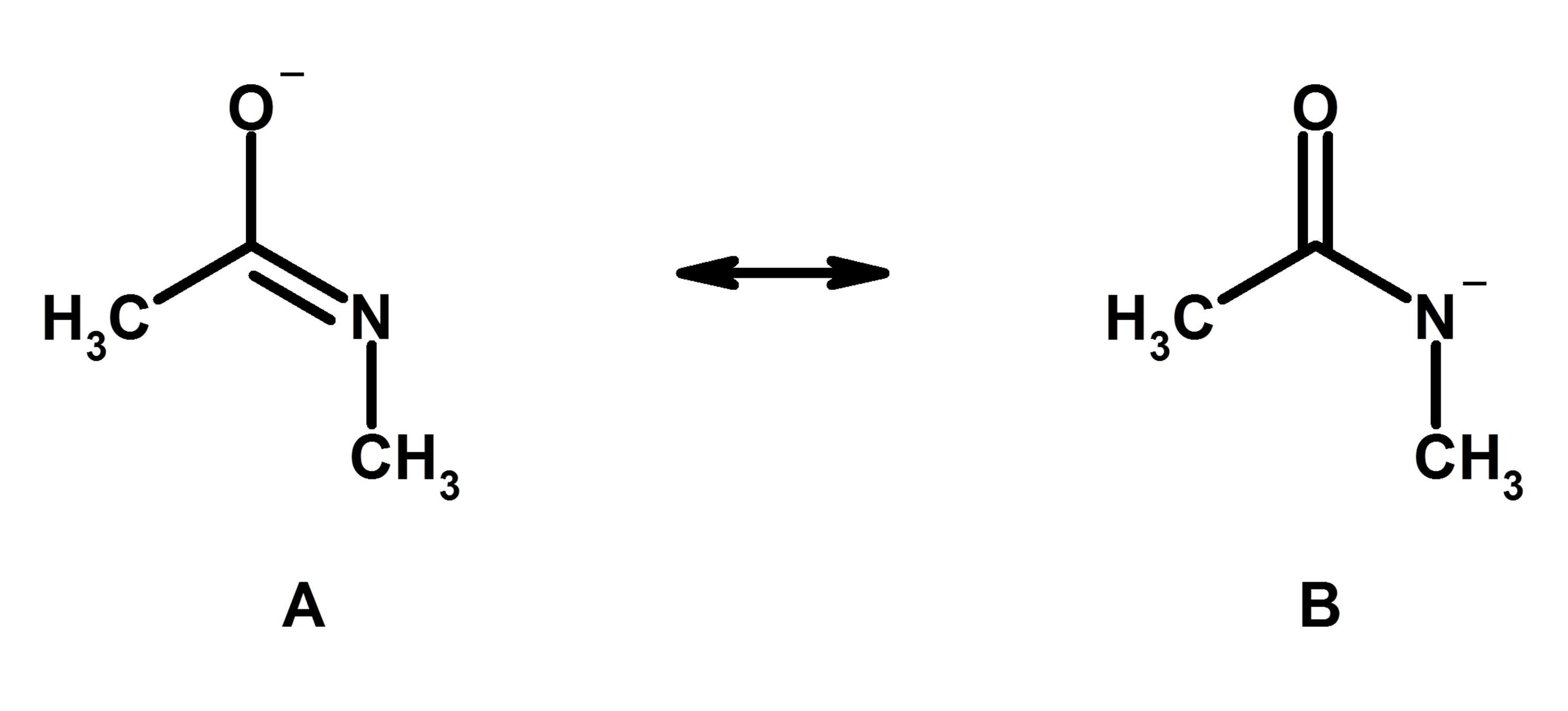
Trick 11 Master Organic Chemistry Resonance Structures
However, A Single Lewis Structure Cannot Explain Chemical Bonding Due To Partial Charges On The Atoms And Fractional Bond Order.
Web Draw The Resonance Structures Of Molecules Or Ions That Exhibit Delocalization.
341K Views 11 Years Ago.
Web The Discussion Of Resonance Effect Heavily Relies On The Understanding Of Resonance Structures.
Related Post: