How To Draw Resonance Hybrids
How To Draw Resonance Hybrids - Get a 10 bullets summary of the topic. Showing 1 of 10 videos. This movement of the electrons is called delocalization. It explains how to identify the major resonance contributor as well as the minor. Web draw the resonance structures of molecules or ions that exhibit delocalization. That lone pair is participating in resonance, which makes this nitrogen sp two hybridized, so it has a p orbital. Draw curved arrows to show the movement of electrons. We say that the best or actual distribution (most stable) is a resonance hybrid of the contributing lewis representations. Resonance is a mental exercise and method within the valence bond theory of bonding that describes the delocalization of. The overall electronic structure of the molecule or ion is given by the weighted average of these resonance structures and is referred to as the resonance hybrid. 84k views 9 years ago orgo basics. Confirm that no rules of. Web this general chemistry video tutorial provides a basic introduction into resonance structures. Rules for drawing contributing structures. Curved arrows communicate electron flow (movement) Resonance is a mental exercise and method within the valence bond theory of bonding that describes the delocalization of. These structures are called resonance structures or contributing structures. The structure is at all times a single resonance hybrid of all the structures. Web this organic chemistry video tutorial explains how to draw resonance structures, how to identify the major resonance. Draw the lewis structure & resonance for the molecule (using solid lines for bonds). Web draw the resonance structures of molecules or ions that exhibit delocalization. That lone pair is participating in resonance, which makes this nitrogen sp two hybridized, so it has a p orbital. Where there can be a double or triple bond, draw a dotted. And then. Confirm that no rules of. Some resonance structures are more favorable than others. Web so what we do for this is we literally combine the two different resonance structures in tow one drawing or 234 etcetera, and we combine them all into one drawing. The actual structure isn't part of the time one structure and part of the time another. Confirm that no rules of. Web when learning to draw and interpret resonance structures, there are a few basic guidelines to help. Web so what we do for this is we literally combine the two different resonance structures in tow one drawing or 234 etcetera, and we combine them all into one drawing. We can draw two or more lewis. Curved arrows communicate electron flow (movement) Resonance is used to represent all the different ways that identical molecules can distribute electrons. The overall electronic structure of the molecule or ion is given by the weighted average of these resonance structures and is referred to as the resonance hybrid. An animation of how one can do a resonance with ozone by. Web the net sum of valid resonance structures is defined as a resonance hybrid, which represents the overall delocalization of electrons within the molecule. This movement of the electrons is called delocalization. In resonance structures, the electrons are able to move to help stabilize the molecule. Web this organic chemistry video tutorial provides a basic introduction into drawing resonance structures.. That lone pair is participating in resonance, which makes this nitrogen sp two hybridized, so it has a p orbital. An animation of how one can do a resonance with ozone by moving electrons. Rules for drawing contributing structures. Web and so, here's a situation where drawing a resonance structure helps clue us into what's actually happening: Web when learning. Web 3 min read. Rules for drawing contributing structures. William reusch, professor emeritus ( michigan state u. Web draw the resonance structures of molecules or ions that exhibit delocalization. Showing 1 of 10 videos. It explains how to draw the resonance structures using curved. A molecule that has several resonance structures is more stable than one with fewer. Web resonance arises when more than one valid lewis structure can be drawn for a molecule or ion. 84k views 9 years ago orgo basics. Understand the rules of resonance and identify where electrons can flow. Web when learning to draw and interpret resonance structures, there are a few basic guidelines to help. Let’s see if we can correlate these drawing conventions to a valence bond theory picture of the bonding in a carboxylate group. Use the concept of resonance to explain structural features of molecules and ions. Web this general chemistry video tutorial provides a basic introduction into resonance structures. And then we try to analyze, which would be the the resident structure that. Confirm that no rules of. Understand the rules of resonance and identify where electrons can flow to or away from. A molecule that has several resonance structures is more stable than one with fewer. Web the electrons aren't travelling at all. It explains how to draw the resonance structures using curved. Draw the lewis structure & resonance for the molecule (using solid lines for bonds). Get a 10 bullets summary of the topic. Web this organic chemistry video tutorial explains how to draw resonance structures, how to identify the major resonance contributor, and how to draw the resonance hybrid. It explains how to identify the major resonance contributor as well as the minor. Craig beals shares his set of rules for drawing lewis structures of molecules that have resonance hybrids in chemistry. These structures are called resonance structures or contributing structures.
Drawing Resonance Hybrids A\L Chemistry YouTube
14.4 The Resonance Hybrid Chemistry LibreTexts
Resonance Hybrid
Resonance Structures 4 Rules On How To Evaluate Them, With Practice

Resonance Structures, Basic Introduction How To Draw The Resonance

Write the resonance structures of CO3^2 and HCO3^

Resonance Chemistry LibreTexts
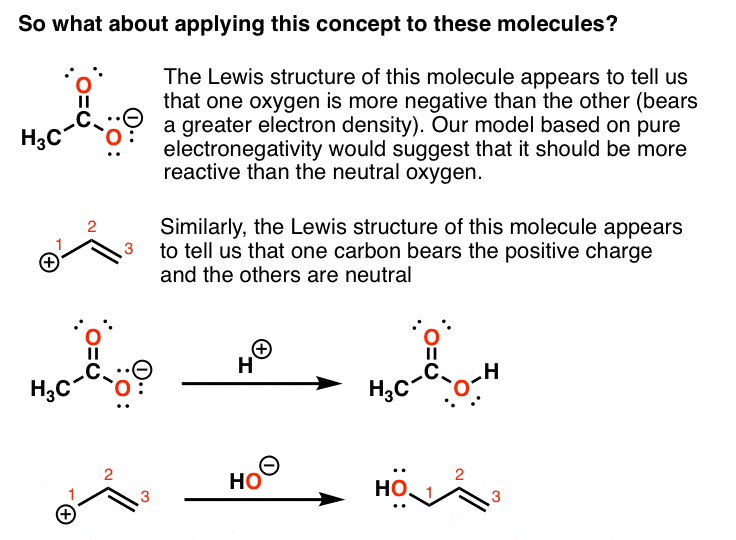
Intro to Resonance In Organic Chemistry Master Organic Chemistry
[Solved] Chemistry Question. 2.29 Draw a resonance hybrid for each of
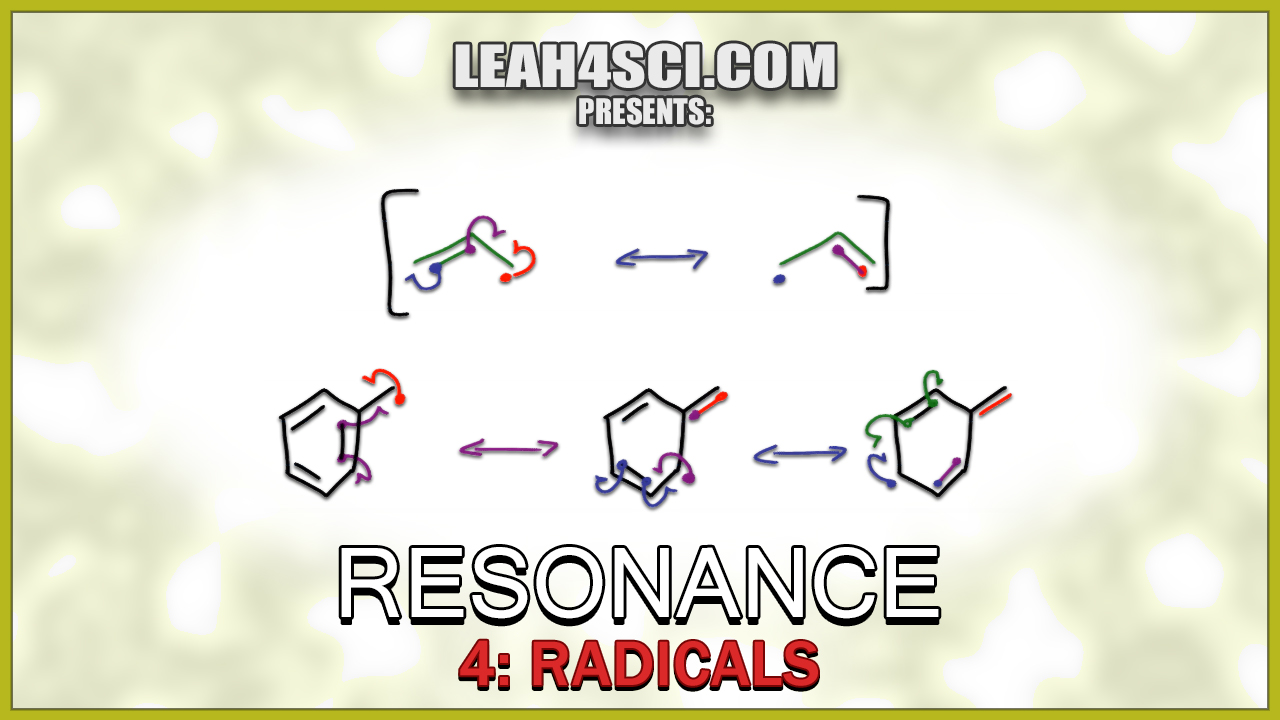
Drawing Radical Resonance for Allylic and Benzylic Radicals Tutorial Video
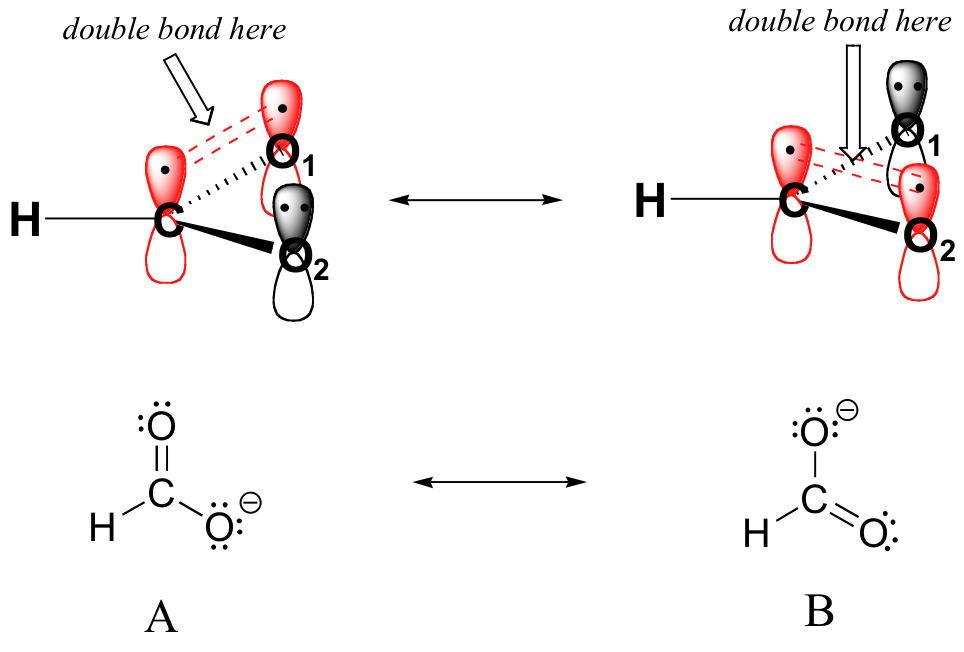
That Lone Pair Is Participating In Resonance, Which Makes This Nitrogen Sp Two Hybridized, So It Has A P Orbital.
We Say That The Best Or Actual Distribution (Most Stable) Is A Resonance Hybrid Of The Contributing Lewis Representations.
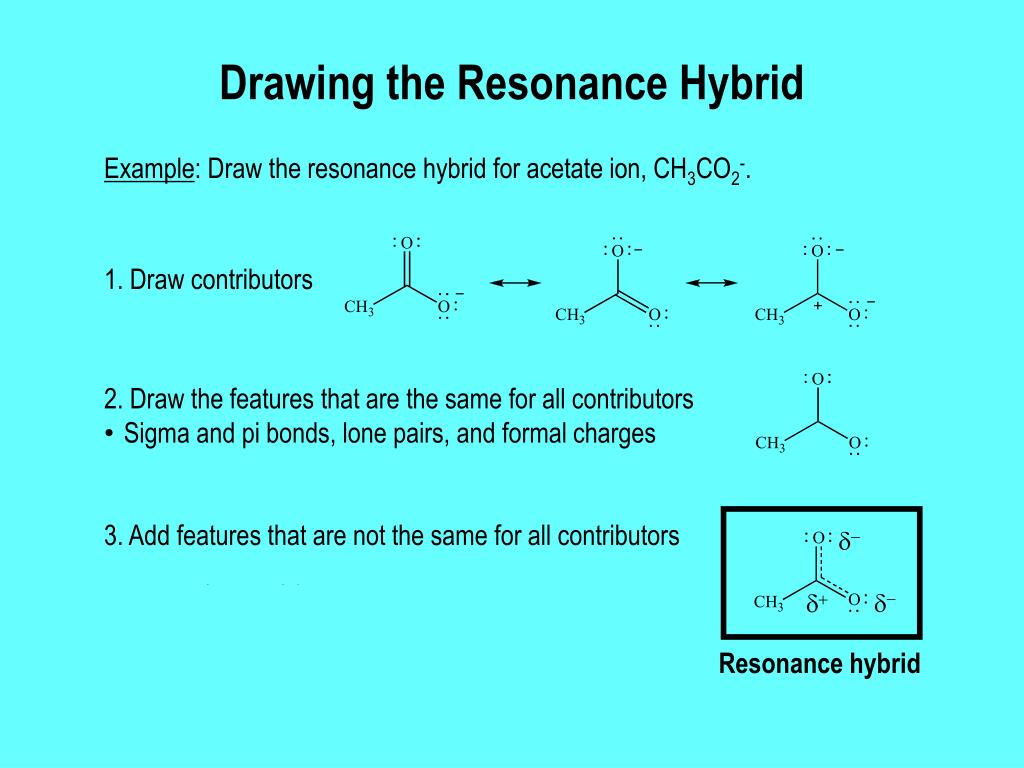
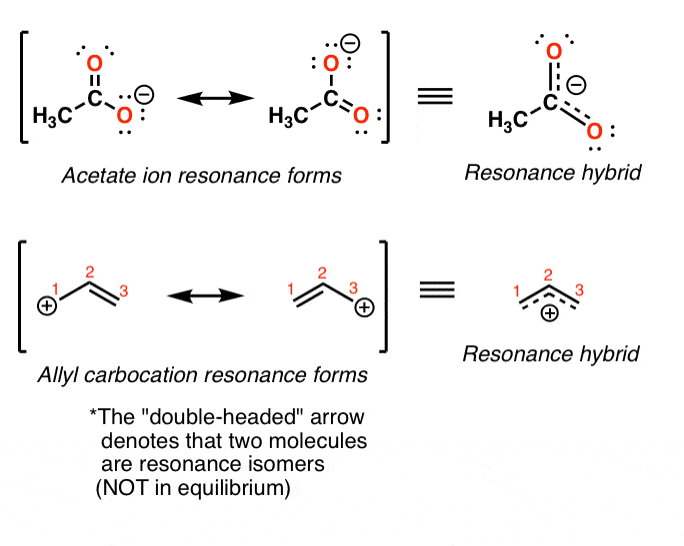
We Can Draw Two Or More Lewis Structures For Some Molecules And Polyatomic Ions, Without Changing The Position Of Atoms In The Structure.
Resonance Is A Mental Exercise And Method Within The Valence Bond Theory Of Bonding That Describes The Delocalization Of.
Related Post: