How To Draw Molecular Diagram
How To Draw Molecular Diagram - During this course, you will view molecules written in all three forms. When constructing a lewis diagram, keep in mind the octet rule, which refers to the tendency of atoms. Although more complex, these diagrams reveal a more realistic case for bonding, allowing electrons to travel about a molecule, rather than in between one. A molecular orbital diagram, or mo diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in. Web construct mo diagrams for simple diatomic molecules and/or compounds. The diagram is also called a lewis dot diagram, lewis dot formula, or electron dot diagram. How do you populate the electrons? Web molecular orbital diagrams. Molview consists of two main parts, a structural formula editor and a 3d model viewer. Web a lewis structure is a diagram that shows the chemical bonds between atoms in a molecule and the valence electrons or lone pairs of electrons. When constructing a lewis diagram, keep in mind the octet rule, which refers to the tendency of atoms. How does this diagram account for the paramagnetism of o 2? Web construct mo diagrams for simple diatomic molecules and/or compounds. How do you populate the electrons? 673k views 5 years ago lewis structure tutorial. Solution we draw a molecular orbital energy diagram similar to that shown in figure 8.37. Web molecular orbital diagrams are complex, involving two additional orbitals, electronegativity, atomic symmetries and atomic energies. How do you populate the electrons? How to draw a molecular orbital diagram. Once you’ve drawn a molecule, you can click the 2d to 3d button to convert the. How do you populate the electrons? Molecular orbital diagrams are diagrams of molecular orbital (mo) energy levels, shown as short horizontal lines in the center, flanked by constituent atomic orbital (ao) energy levels for comparison, with the energy levels increasing from the bottom to the top. These steps may then be extrapolated to construct more difficult polyatomic diagrams. We saw. Web drawing molecular orbital diagrams is one of the trickier concepts in chemistry. This reduces to:γσ= a1g+ eg+ t1usix gosin total. Now let's think about how to make some slightly more complicated mo diagrams. Make reducible reps for sigma bond vectors. This is a whiteboard animation tutorial on how to draw lewis structures of molecules. Now let's think about how to make some slightly more complicated mo diagrams. Solution we draw a molecular orbital energy diagram similar to that shown in figure 8.37. Web molecular orbital diagrams. These steps may then be extrapolated to construct more difficult polyatomic diagrams. A molecular orbital diagram, or mo diagram, is a qualitative descriptive tool explaining chemical bonding in. Web molecular orbital diagrams, bond order, and number of unpaired electrons draw the molecular orbital diagram for the oxygen molecule, o 2. The diagram is also called a lewis dot diagram, lewis dot formula, or electron dot diagram. Web molecular orbital diagrams. Web a molecular orbital diagram for a diatomic molecule (two atoms) varies in the number of electrons. 192. Molecular orbital diagrams are diagrams of molecular orbital (mo) energy levels, shown as short horizontal lines in the center, flanked by constituent atomic orbital (ao) energy levels for comparison, with the energy levels increasing from the bottom to the top. Determine the atomic orbitals of your atoms. Molview consists of two main parts, a structural formula editor and a 3d. Now let's think about how to make some slightly more complicated mo diagrams. Shared pairs of electrons are drawn as lines between atoms, while lone pairs of electrons are drawn as dots next to atoms. These steps may then be extrapolated to construct more difficult polyatomic diagrams. During this course, you will view molecules written in all three forms. Web. Make reducible reps for sigma bond vectors. 673k views 5 years ago lewis structure tutorial. This reduces to:γσ= a1g+ eg+ t1usix gosin total. A molecular orbital diagram, or mo diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in. That's the number of valence electrons on. 192 views 9 months ago explanation. Web molecular orbital diagrams, bond order, and number of unpaired electrons draw the molecular orbital diagram for the oxygen molecule, o 2. Web the objective of this wiki is to provide readers with the fundamental steps in constructing simple homonuclear and heteronuclear diatomic molecular orbital diagrams. Web molecular orbital diagrams. That's the number of. This reduces to:γσ= a1g+ eg+ t1usix gosin total. How does this diagram account for the paramagnetism of o 2? Molecular orbital diagrams are diagrams of molecular orbital (mo) energy levels, shown as short horizontal lines in the center, flanked by constituent atomic orbital (ao) energy levels for comparison, with the energy levels increasing from the bottom to the top. Solution we draw a molecular orbital energy diagram similar to that shown in figure 8.37. Shared pairs of electrons are drawn as lines between atoms, while lone pairs of electrons are drawn as dots next to atoms. 192 views 9 months ago explanation. Determine the atomic orbitals of your atoms. The first major step is understanding the difference between two major theories: Web a molecular orbital diagram for a diatomic molecule (two atoms) varies in the number of electrons. Web a lewis structure is a diagram that shows the chemical bonds between atoms in a molecule and the valence electrons or lone pairs of electrons. During this course, you will view molecules written in all three forms. We saw two simple mo diagrams in the section on h 2. This is a whiteboard animation tutorial on how to draw lewis structures of molecules. The diagram is also called a lewis dot diagram, lewis dot formula, or electron dot diagram. Web construct mo diagrams for simple diatomic molecules and/or compounds. When constructing a lewis diagram, keep in mind the octet rule, which refers to the tendency of atoms.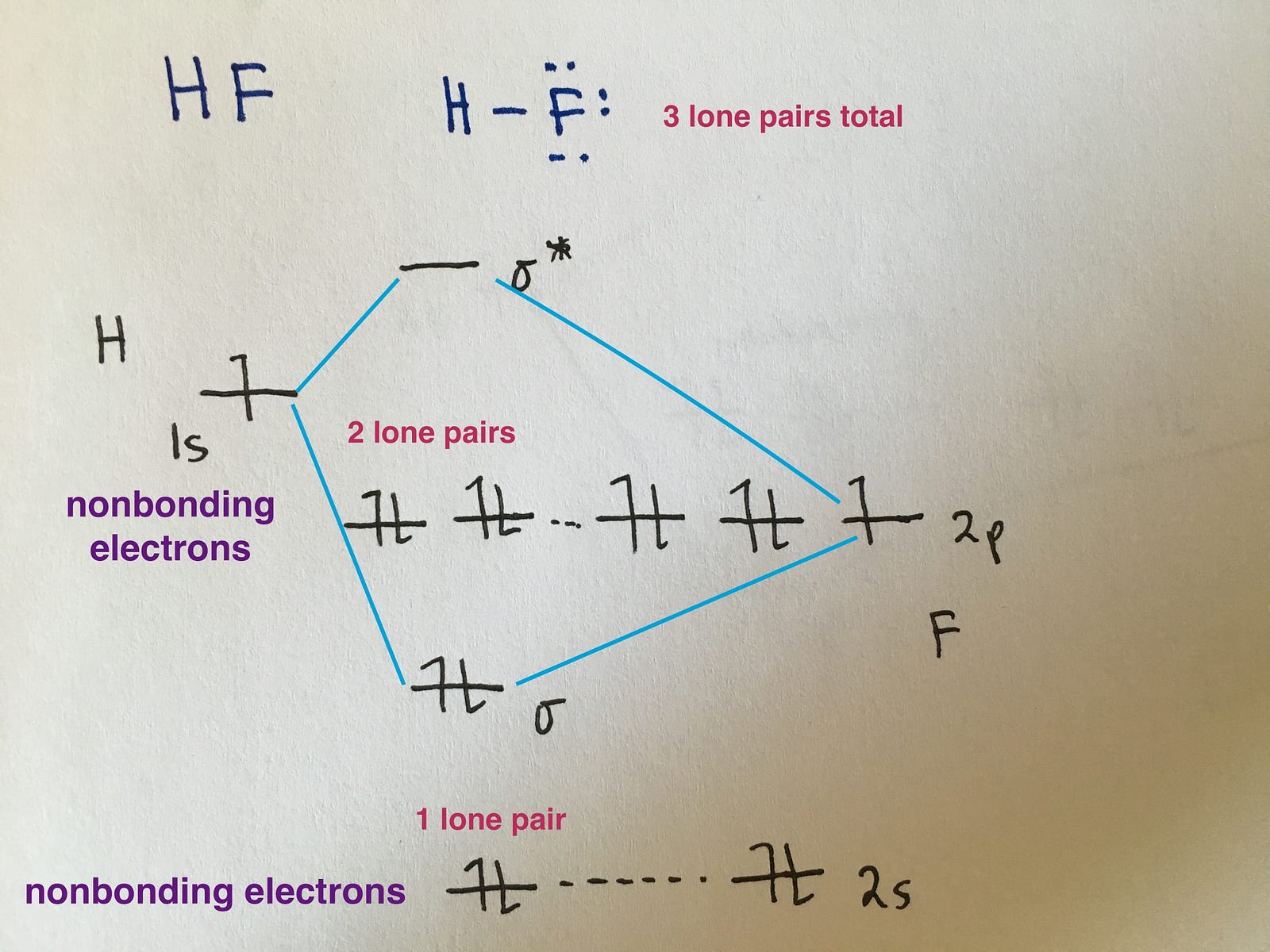
Molecular Orbital Diagrams simplified Megan Lim Medium

how to draw molecular orbital diagrams for polyatomic molecules cub
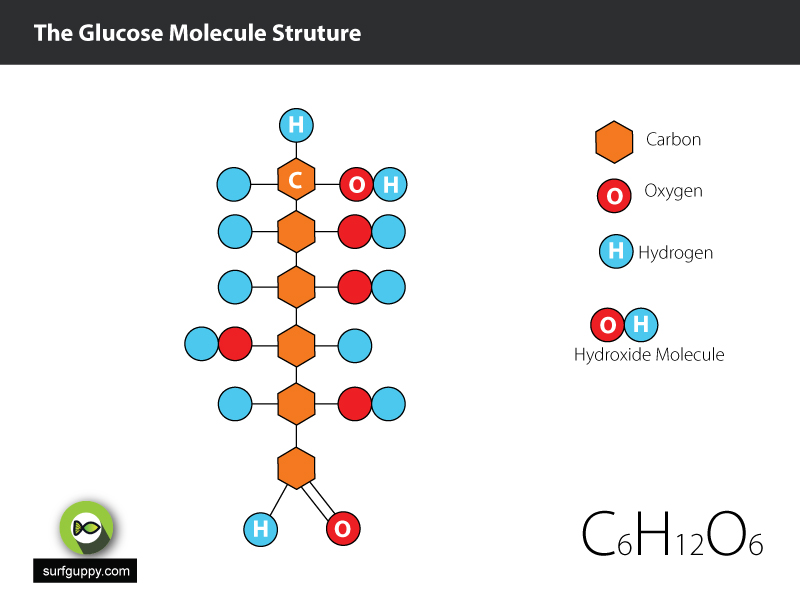
4 simple steps to drawing chain structure of glucose molecule
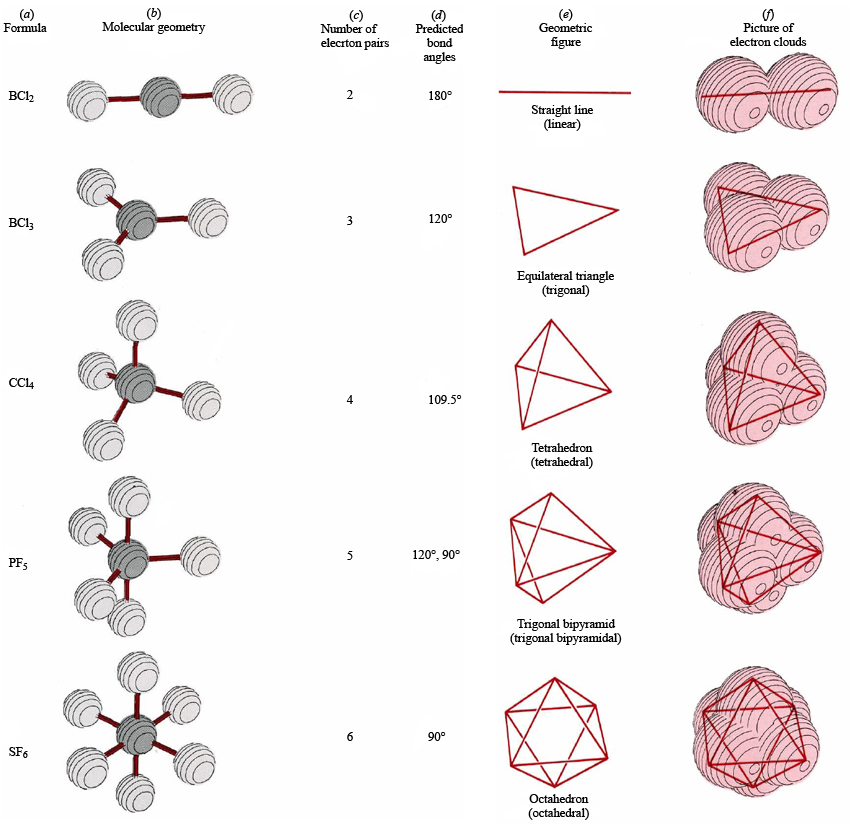
Molecular Geometry Chemistry Socratic
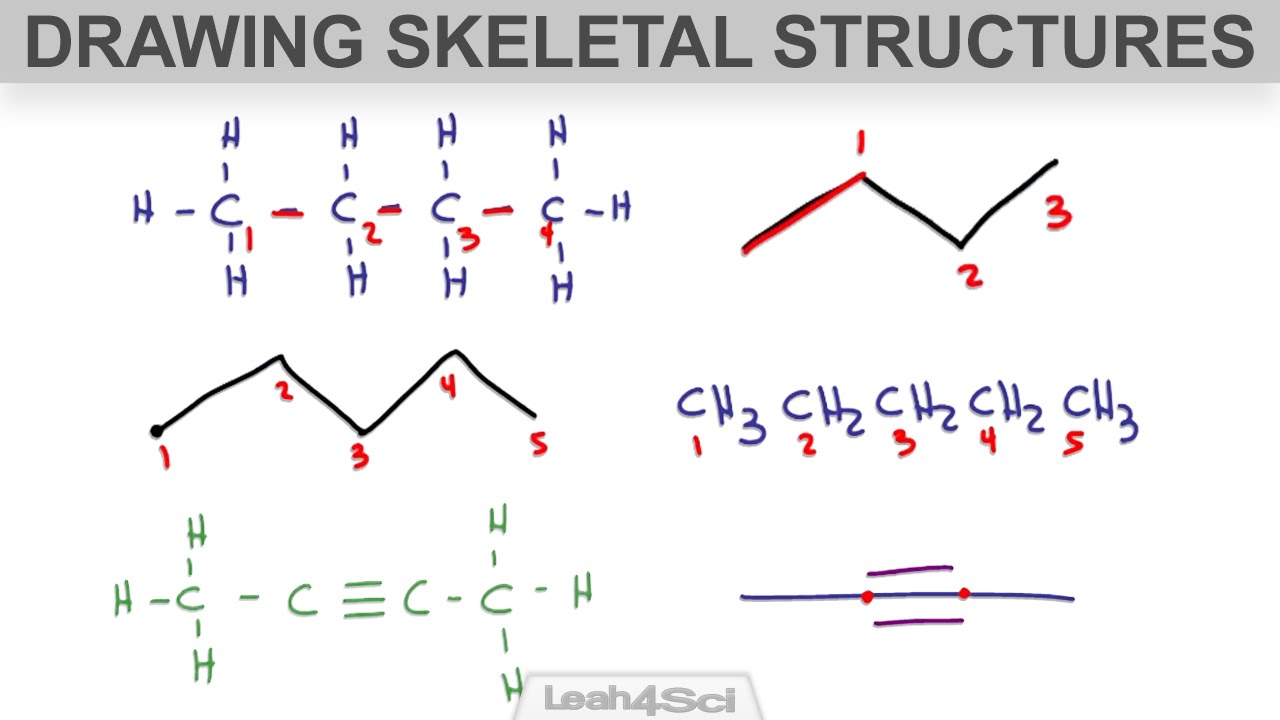
How to Draw Skeletal Structure or BondLine Notation for Organic
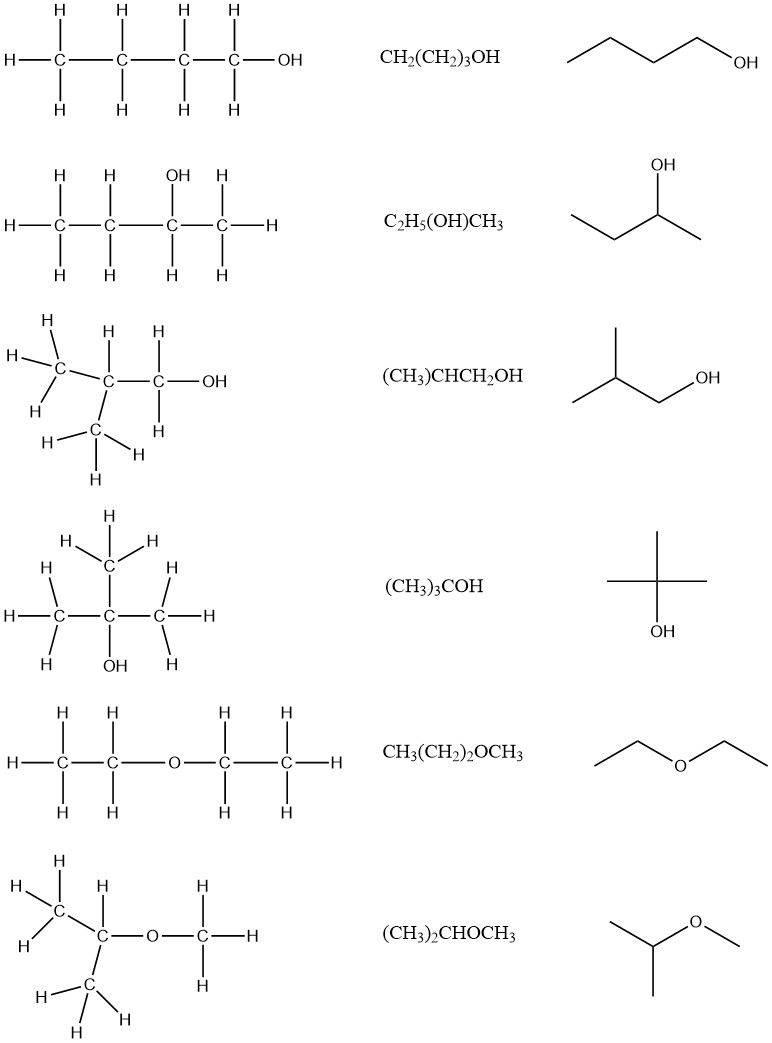
1.13 Drawing Chemical Structures Chemistry LibreTexts

Molecular Modeling Digital and Analog General Chemistry Lab News
Chemical structure Using illustrations to explain chemistry Mind the

How to search for a chemical molecule by drawing its chemical structure

Molecular Orbital Diagrams simplified by Megan Lim Medium
These Steps May Then Be Extrapolated To Construct More Difficult Polyatomic Diagrams.
How Do You Populate The Electrons?
A Lewis Diagram Shows How The Valence Electrons Are Distributed Around The Atoms In A Molecule.
Although More Complex, These Diagrams Reveal A More Realistic Case For Bonding, Allowing Electrons To Travel About A Molecule, Rather Than In Between One.
Related Post: