How To Draw Isotopes
How To Draw Isotopes - Web the average atomic mass of an element is a weighted average calculated by multiplying the relative abundances of the element's isotopes by their atomic masses and then. Calculate atomic weight from percent abundance. How can i plot gmwl, lmwl and and evaporation line using stable isotopes values? Web they then draw conclusions for the system across a wider range of conditions. Determine the number of protons, neutrons, and electrons in a specific isotope. Web carbon has 2 electrons in its first shell and 4 in its second shell.check me out: You put the atomic number, mass number, and net charge around the chemical. While some students like manipulating solid objects, others prefer drawing. A tip ( tip #1) that i find useful when drawing isomers is to. Manipulate the atomic weight equation to calculate various unknown variables. Web 415k views 12 years ago. You put the atomic number, mass number, and net charge around the chemical. Identify the element and write symbols for the isotopes. Determine the number of protons, neutrons, and electrons in a specific isotope. While some students like manipulating solid objects, others prefer drawing. Mass versus time graph, with the mass decreasing exponentially over time. In this case, we’re going to use c 6 h 14. Atoms of the same element with different numbers of neutrons are called. You put the atomic number, mass number, and net charge around the chemical. Web an element with three stable isotopes has 82 protons. Isotopes of an element have: Web describe the location, charge, and relative mass of the neutron. You should do so only if this showme contains inappropriate. Are you sure you want to remove this showme? However, physicists have come to understand in recent years that an eos obtained from an. Isotopes of an element have: Check out the next lesson and practice what you’re. Web isotopes are atoms of the same element that have the same number of protons (i.e., atomic number, z) but a different number of neutrons, meaning that their mass number, a,. How can i plot gmwl, lmwl and and evaporation line using stable isotopes values? You. Web the best way to explain how to draw an isomer is to use an example. Web in isotope notation, you can quickly show how many protons, neutrons, and electrons are in an atom. Because they contain different numbers of neutrons, isotopes have different atomic masses. Web carbon has 2 electrons in its first shell and 4 in its second. Web they then draw conclusions for the system across a wider range of conditions. Check out the next lesson and practice what you’re. In this case, we’re going to use c 6 h 14. Web how can i plot stable isotopes? Web the average atomic mass of an element is a weighted average calculated by multiplying the relative abundances of. Web the best way to explain how to draw an isomer is to use an example. Define isotope and mass number. Web another way to represent isotopes, let's say we wanted to represent this isotope in a different way, sometimes you'll see it where you write the name of the element. In this case, we’re going to use c 6. Identify the element and write symbols for the isotopes. Atomic number the number of protons in the nucleus of an. Web they then draw conclusions for the system across a wider range of conditions. Web isotopes are atoms of the same element that have the same number of protons (i.e., atomic number, z) but a different number of neutrons, meaning. Define isotope and mass number. Web how can i plot stable isotopes? Atoms of the same element with different numbers of neutrons are called. Determine the number of protons, neutrons, and electrons in a specific isotope. Web isotope notation, also known as nuclear notation, is important because it allows us to use a visual symbol to easily determine an isotope's. How can i plot gmwl, lmwl and and evaporation line using stable isotopes values? We’ll examine how we use mass spectrometry to precisely measure the mass and abundance of a given element’s isotopes. Identify the element and write symbols for the isotopes. Mass versus time graph, with the mass decreasing exponentially over time. Web the best way to explain how. Web isotopes are atoms of the same element that have the same number of protons (i.e., atomic number, z) but a different number of neutrons, meaning that their mass number, a,. Web describe the location, charge, and relative mass of the neutron. Web how can i plot stable isotopes? By default, chemdraw correctly recognizes all isotopes in the full table of the elements. 722k views 6 years ago new ap & general chemistry video playlist. Are you sure you want to remove this showme? Calculate atomic weight from percent abundance. Atoms of the same element with different numbers of neutrons are called. Web an element with three stable isotopes has 82 protons. Web isotope notation, also known as nuclear notation, is important because it allows us to use a visual symbol to easily determine an isotope's mass number, atomic number, and to. Because they contain different numbers of neutrons, isotopes have different atomic masses. Atomic number the number of protons in the nucleus of an. Web 415k views 12 years ago. The separate isotopes contain 124, 125, and 126 neutrons. Mass versus time graph, with the mass decreasing exponentially over time. We’ll examine how we use mass spectrometry to precisely measure the mass and abundance of a given element’s isotopes.
Isotopes Time Scavengers

Atom and molecules class 9 notes ISOTOPES
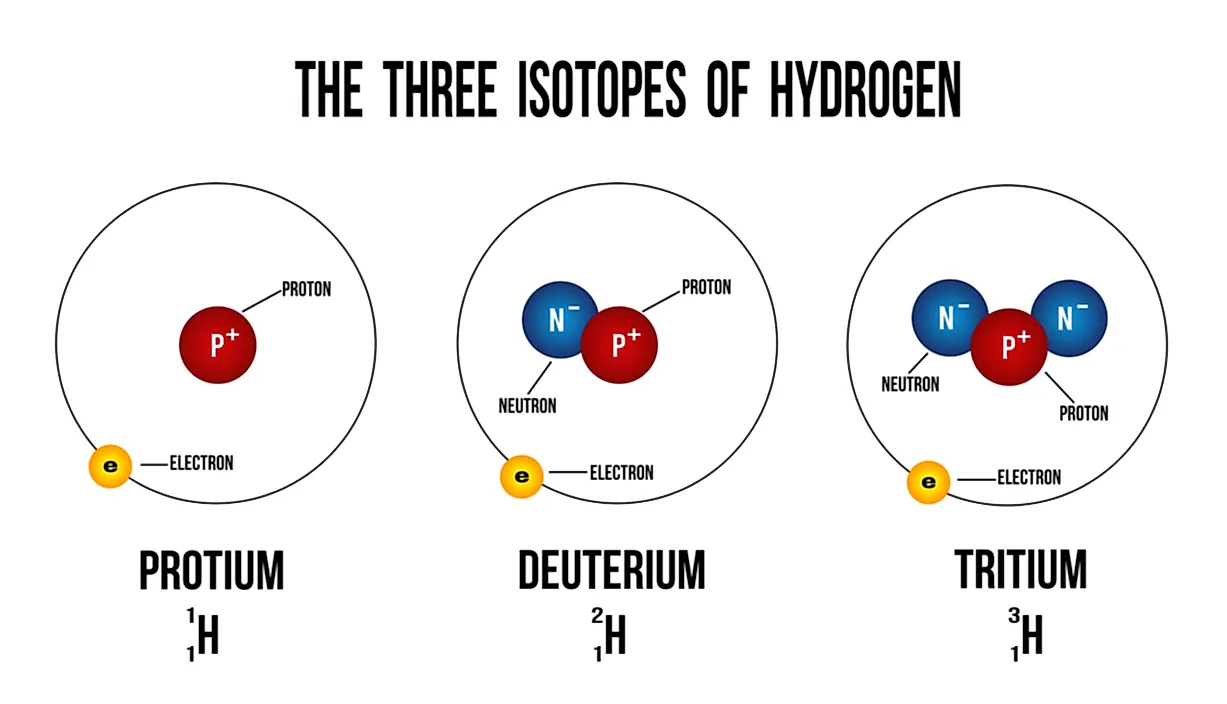
What is an Isotope? The Knowledge Library

Isotopes and Isobars Definition, Uses and Difference Teachoo
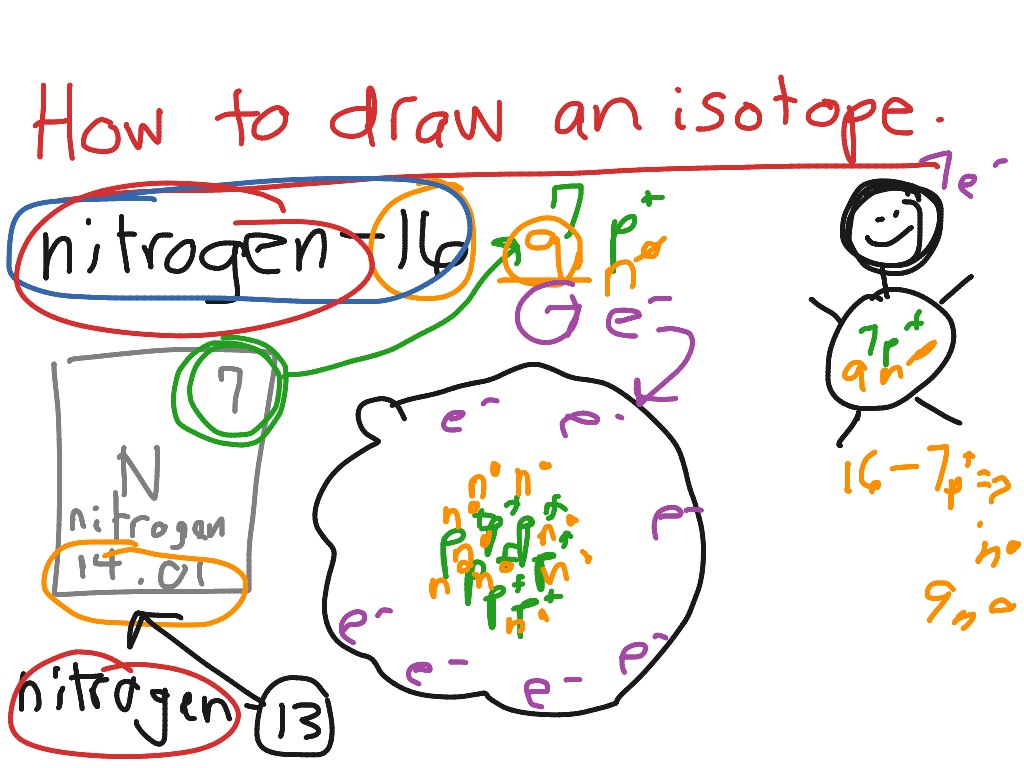
How to Draw an Isotope Science ShowMe

PPT Isotopes PowerPoint Presentation, free download ID2481748

Isotopes What are Isotopes? Relative Atomic Mass
/Isotope-58dd6b415f9b5846830254ae.jpg)
Isotopes Definition and Examples in Chemistry

How to draw an isotope YouTube
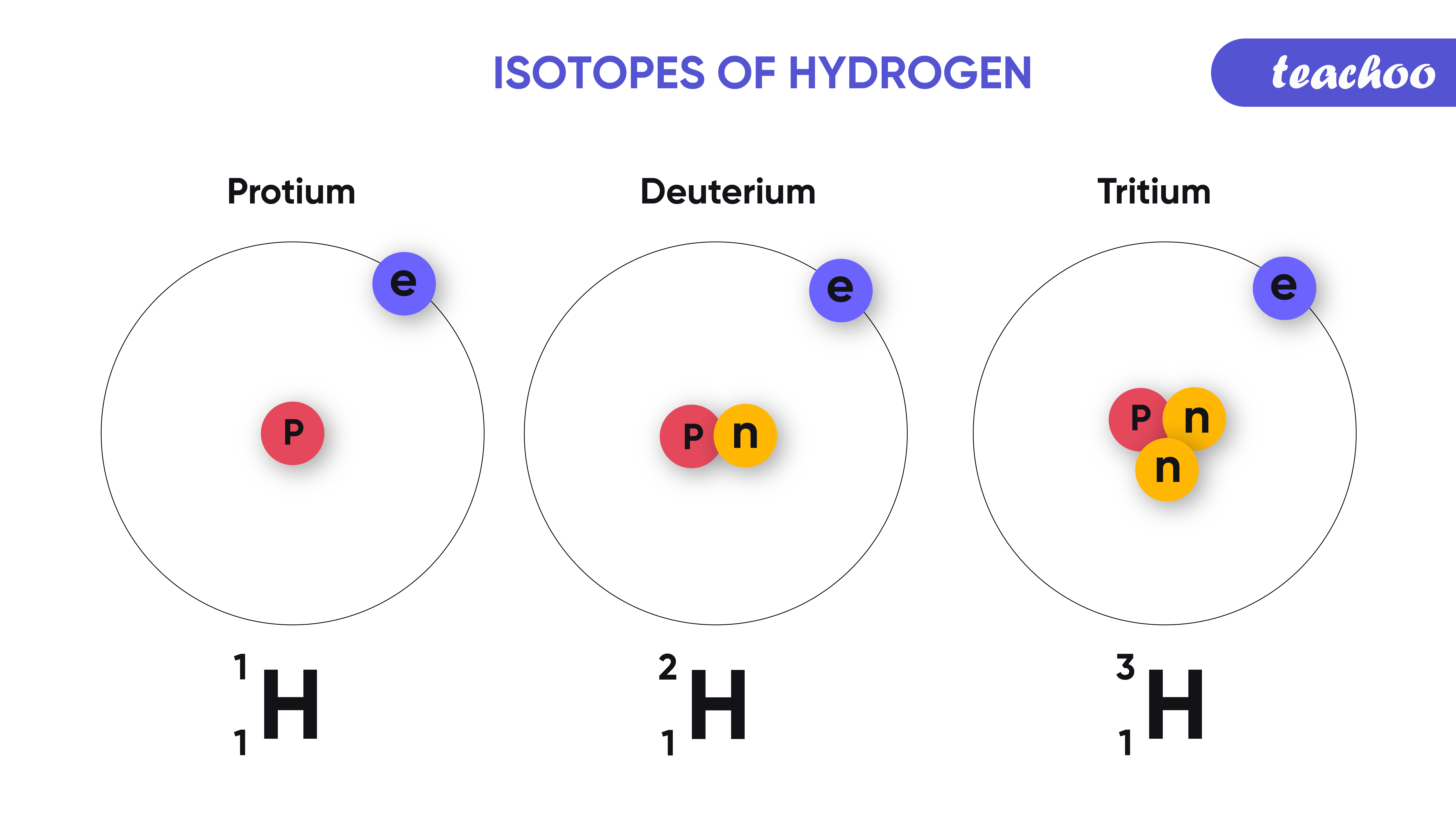
Isotopes and Isobars Definition, Uses and Difference Teachoo
Web About Press Copyright Contact Us Creators Advertise Developers Terms Privacy Policy & Safety How Youtube Works Test New Features Nfl Sunday Ticket Press Copyright.
Web Isotopes Are Atoms Of The Same Element With Different Numbers Of Neutrons.
You Should Do So Only If This Showme Contains Inappropriate.
Check Out The Next Lesson And Practice What You’re.
Related Post: