How To Draw Hydrogen
How To Draw Hydrogen - H ν = δ e = ( 1 n l o w 2 − 1 n h i g h 2) ⋅ 13.6 ev. 1) find the number of valence electrons, find the electronegativity of the elements, and consider the type of bonding. Web if you were to draw every carbon hydrogen bond in organic chemistry class it would take you forever. Web what are the steps for drawing lewis dot diagrams? Each oxygen atom has two bonds. A lewis diagram shows how the valence electrons are distributed around the atoms in a molecule. Each hydrogen atom has one bond. Web 5.5k views 8 years ago. A very simple drawing of an hydrogen atom with a nucleus and an electron :).more. Shared pairs of electrons are drawn as lines between atoms, while lone pairs of electrons are drawn as dots next to atoms. Web bohr's model of hydrogen is based on the nonclassical assumption that electrons travel in specific shells, or orbits, around the nucleus. Each nitrogen atom has three bonds. Carbon is still bonded to these hydrogens but we're going to ignore them for our bond line structure. Web the commonest way to draw structural formulae. Web what are the steps for. Web to draw the lewis structure of an atom, write the symbol of the atom and draw dots around it to represent the valence electrons. Web when drawing the structure of a neutral organic compound, you will find it helpful to remember that. H ν = δ e = ( 1 n l o w 2 − 1 n h. A very simple drawing of an hydrogen atom with a nucleus and an electron :).more. 1) find the number of valence electrons, find the electronegativity of the elements, and consider the type of bonding. Web what are the steps for drawing lewis dot diagrams? In this video we'll look at the atomic structure and bohr model for the hydrogen atom. You can simplify the formula by writing, for example, ch 3 or ch 2 instead of showing all these bonds. For example, ethanoic acid would be shown in a. 2) draw the atoms as. Also, helium is shown in group 8a, but it only has two valence electrons. Web the commonest way to draw structural formulae. 1) find the number of valence electrons, find the electronegativity of the elements, and consider the type of bonding. Each hydrogen atom has one bond. Web when drawing the structure of a neutral organic compound, you will find it helpful to remember that. 2) draw the atoms as. Shared pairs of electrons are drawn as lines between atoms, while lone. E ( n) = − 1 n 2 ⋅ 13.6 ev. In this video we'll look at the atomic structure and bohr model for the hydrogen atom (h). Carbon is still bonded to these hydrogens but we're going to ignore them for our bond line structure. Each carbon atom has four bonds. Web 5.5k views 8 years ago. Web if you were to draw every carbon hydrogen bond in organic chemistry class it would take you forever. So, we leave those out in bond line structures. Shared pairs of electrons are drawn as lines between atoms, while lone pairs of electrons are drawn as dots next to atoms. 2) draw the atoms as. In this video we'll look. You can simplify the formula by writing, for example, ch 3 or ch 2 instead of showing all these bonds. E ( n) = − 1 n 2 ⋅ 13.6 ev. Each carbon atom has four bonds. H ν = δ e = ( 1 n l o w 2 − 1 n h i g h 2) ⋅ 13.6. Web to draw the lewis structure of an atom, write the symbol of the atom and draw dots around it to represent the valence electrons. Web the commonest way to draw structural formulae. Web bohr's model of hydrogen is based on the nonclassical assumption that electrons travel in specific shells, or orbits, around the nucleus. H ν = δ e. Also, helium is shown in group 8a, but it only has two valence electrons. Web if you were to draw every carbon hydrogen bond in organic chemistry class it would take you forever. Shared pairs of electrons are drawn as lines between atoms, while lone pairs of electrons are drawn as dots next to atoms. Each hydrogen atom has one. Each carbon atom has four bonds. Carbon is still bonded to these hydrogens but we're going to ignore them for our bond line structure. H ν = δ e = ( 1 n l o w 2 − 1 n h i g h 2) ⋅ 13.6 ev. Each hydrogen atom has one bond. 2) draw the atoms as. Also, helium is shown in group 8a, but it only has two valence electrons. So, we leave those out in bond line structures. 14k views 1 year ago. A very simple drawing of an hydrogen atom with a nucleus and an electron :).more. Web what are the steps for drawing lewis dot diagrams? Web the commonest way to draw structural formulae. 1) find the number of valence electrons, find the electronegativity of the elements, and consider the type of bonding. Each oxygen atom has two bonds. Web 5.5k views 8 years ago. For example, ethanoic acid would be shown in a. Web if you were to draw every carbon hydrogen bond in organic chemistry class it would take you forever.
Diagram Representation Of The Element Hydrogen Stock Vector Image

How to Learn About the Chemistry of the Hydrogen Atom 12 Steps

Hydrogen atom diagram concept illustration Stock Vector Image & Art Alamy

How To Draw Hydrogen Bonds Askworksheet

Hydrogen Atomic Structure

Describe Bohr’s model of the hydrogen atom. bitWise Academy

Diagram representation element hydrogen Royalty Free Vector

Periodic table hydrogen atomic number millgulf
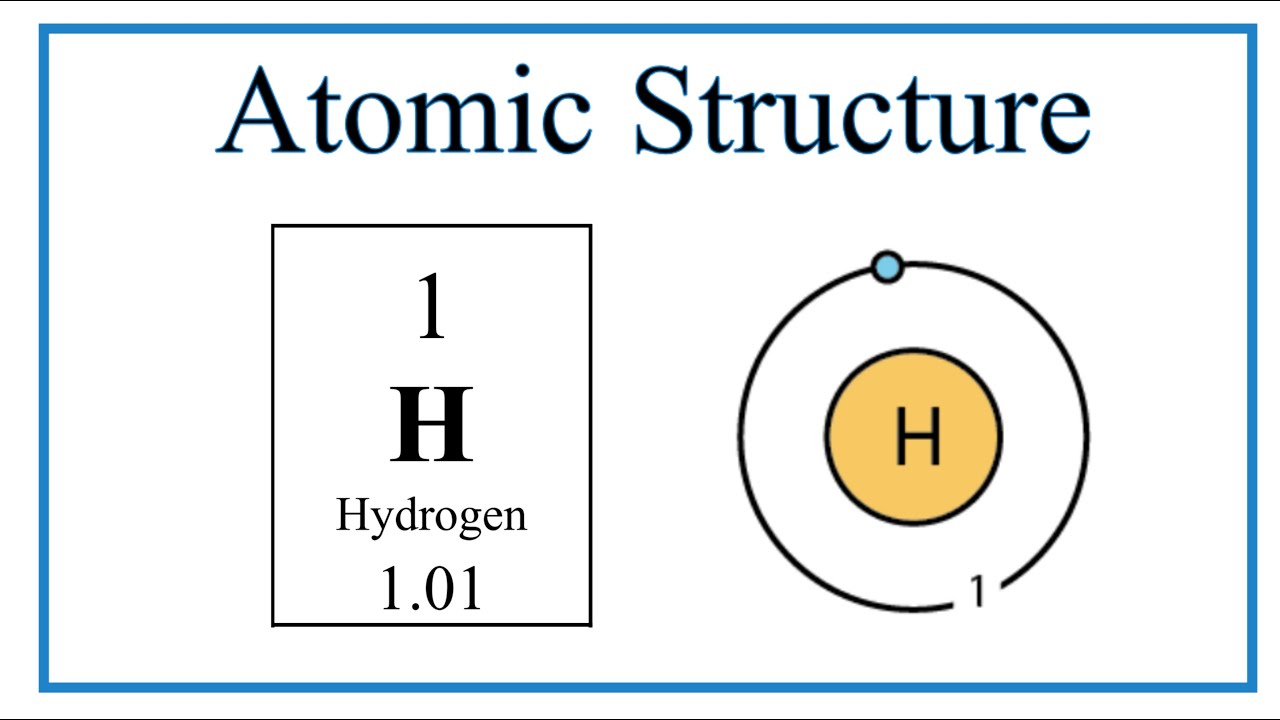
Bohr Atomic Model Of Hydrogen

How to Draw the Lewis Dot Structure for H+ (Hydrogen ion) YouTube
Each Nitrogen Atom Has Three Bonds.
In This Video We'll Look At The Atomic Structure And Bohr Model For The Hydrogen Atom (H).
We’ll Use A Bohr Diagram To.
Web To Draw The Lewis Structure Of An Atom, Write The Symbol Of The Atom And Draw Dots Around It To Represent The Valence Electrons.
Related Post: