How To Draw Hydrogen Bonding
How To Draw Hydrogen Bonding - If you were to draw every carbon hydrogen bond in organic chemistry class it would take you forever. Web learn which types of molecules make hydrogen bonds and how to draw the hydrogen bonding interactions between two molecules of the same type and between two m. Using lewis structures, we can represent this as follows: Notice that each water molecule can potentially form four hydrogen bonds with surrounding water molecules. 4.7k views 7 years ago 1d: Web hydrogen bonding in water (video) | khan academy. 10k views 3 years ago #exploding #teacher #chemical. Hydrogen bonding between different parts of the same chain (intramolecular bonding; The number of lone pairs on the o or n. Web that will prepare the company for the energy transition, putting us in a position to handle fuels like ammonia and even hydrogen in the future because a lot of the gas characteristics are similar. Hydrogen bonds are strong intermolecular forces created when a hydrogen atom bonded to an electronegative atom approaches a nearby electronegative atom. If you were to draw every carbon hydrogen bond in organic chemistry class it would take you forever. 1 pm = 1 × 10 −12 m). Web the bond in a hydrogen molecule, measured as the distance between the. 1.31 explain the formation of simple molecular, covalent substances, using dot and cross diagrams,. Web turning 35 into 4. Each water molecule is hydrogen bonded to four others. 1 pm = 1 × 10 −12 m). The molecules which have this extra bonding are: This particular bond length represents a balance between several forces: If the reactive surface of these 2d materials were made to react with hydrogen, it should be possible to influence. Shared pairs of electrons are drawn as lines between atoms, while lone pairs of electrons are drawn as dots next to atoms. The molecules which have this extra bonding are:. Using lewis structures, we can represent this as follows: Ammonia can form a maximum of one hydrogen bond per molecule. Shared pairs of electrons are drawn as lines between atoms, while lone pairs of electrons are drawn as dots next to atoms. There are exactly the right numbers of + hydrogens and lone pairs so that every one of them. Water is made up of two hydrogens and one oxygen atom, arranged in a tetrahedral shape. The number of lone pairs on the o or n. Science > ap®︎/college biology > chemistry of life > structure of water and hydrogen bonding. Note that each atom must contribute one electron to the bond. The partial negative charge on the o of. Hydrogen bonds are strong intermolecular forces created when a hydrogen atom bonded to an electronegative atom approaches a nearby electronegative atom. Shared pairs of electrons are drawn as lines between atoms, while lone pairs of electrons are drawn as dots next to atoms. Next, let's think about the carbon hydrogen bonds. The solid line represents a bond in the plane. Web water as a perfect example of hydrogen bonding. Web the bond in a hydrogen molecule, measured as the distance between the two nuclei, is about 7.4 × 10 −11 m, or 74 picometers (pm; Hydrogen bonding between different parts of the same chain (intramolecular bonding; If you were to draw every carbon hydrogen bond in organic chemistry class it. Web the bond in a hydrogen molecule, measured as the distance between the two nuclei, is about 7.4 × 10 −11 m, or 74 picometers (pm; The attractions between oppositely charged electrons and nuclei, the repulsion between two negatively charged. Web turning 35 into 4. The number of hydrogen bonds depends on: This is why the boiling point of water. The number of hydrogen atoms attached to o or n in the molecule. Web water molecules forming hydrogen bonds with one another. The solid line represents a bond in the plane of the screen or paper. Web hydrogen bonding in water (video) | khan academy. If you were to draw every carbon hydrogen bond in organic chemistry class it would. 1 pm = 1 × 10 −12 m). Web hydrogen bonding in water (video) | khan academy. Note that each atom must contribute one electron to the bond. The number of hydrogen atoms attached to o or n in the molecule. Atoms can form more than one bond. Such a bond is weaker than an ionic bond or covalent bond but stronger than van der waals forces. Web describe how hydrogen bonding affects the physical properties of molecular systems, give examples of molecular systems where hydrogen bonding is prevalent (e.g., hydration of anions, proteins, and alcohols) and explain the role hydrogen bonding plays in these systems, draw or interpret diagrams and display formulas showing hydrogen bonding. 10k views 3 years ago #exploding #teacher #chemical. If the reactive surface of these 2d materials were made to react with hydrogen, it should be possible to influence. The origin of hydrogen bonding. Note that each atom must contribute one electron to the bond. Web turning 35 into 4. Using lewis structures, we can represent this as follows: The number of hydrogen bonds depends on: 4.7k views 7 years ago 1d: This is why the boiling point of water is higher than. Each water molecule is hydrogen bonded to four others. Two fluorine atoms can form a molecule of f 2 in the same fashion. Web the bond in a hydrogen molecule, measured as the distance between the two nuclei, is about 7.4 × 10 −11 m, or 74 picometers (pm; Ammonia can form a maximum of one hydrogen bond per molecule. Hydrogen bonds are strong intermolecular forces created when a hydrogen atom bonded to an electronegative atom approaches a nearby electronegative atom.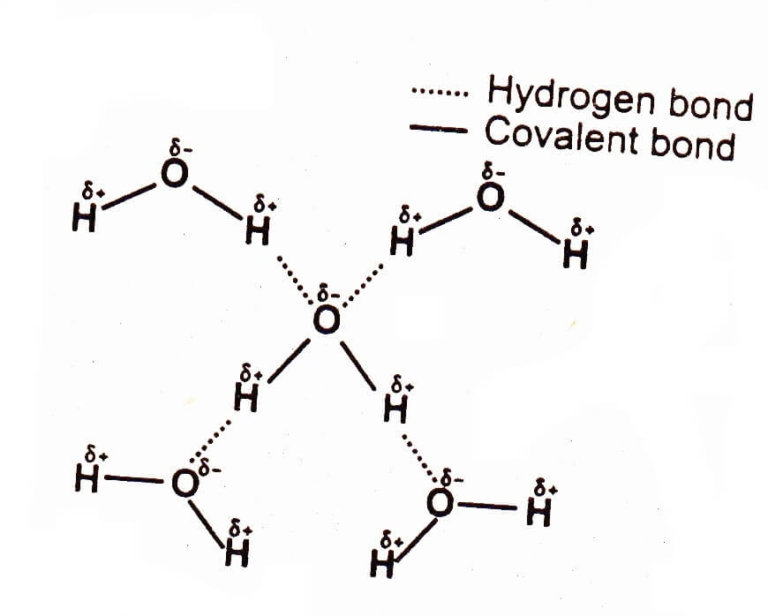
Hydrogen Bonding Chemistry Skills
![[DIAGRAM] Labeled Diagram Of Hydrogen Bonding](http://ffden-2.phys.uaf.edu/webproj/211_fall_2016/Roger_Vang/Roger_Vang/Hydrogen Bonding.png)
[DIAGRAM] Labeled Diagram Of Hydrogen Bonding
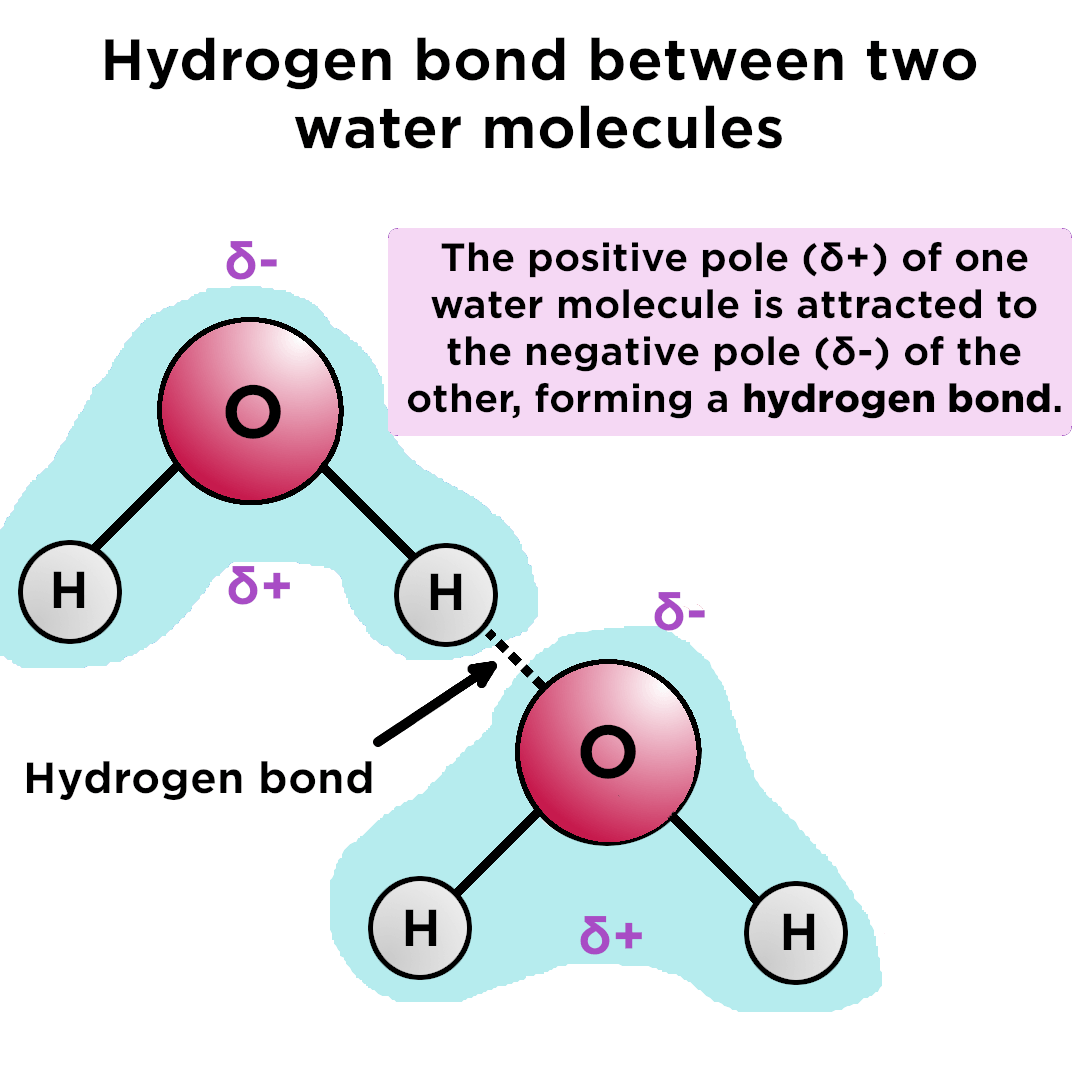
High Specific Heat (Water) — Properties & Examples Expii

How To Draw Hydrogen Bonds Askworksheet

Diagram Of Water Molecules Hydrogen Bonding

Hydrogen Bonding in water Dr. M. Chemistry Tutor

Draw a diagram of water molecules, labeling the hydrogen bond and

How To Draw Hydrogen Bonds

Hydrogen Bonding American Chemical Society
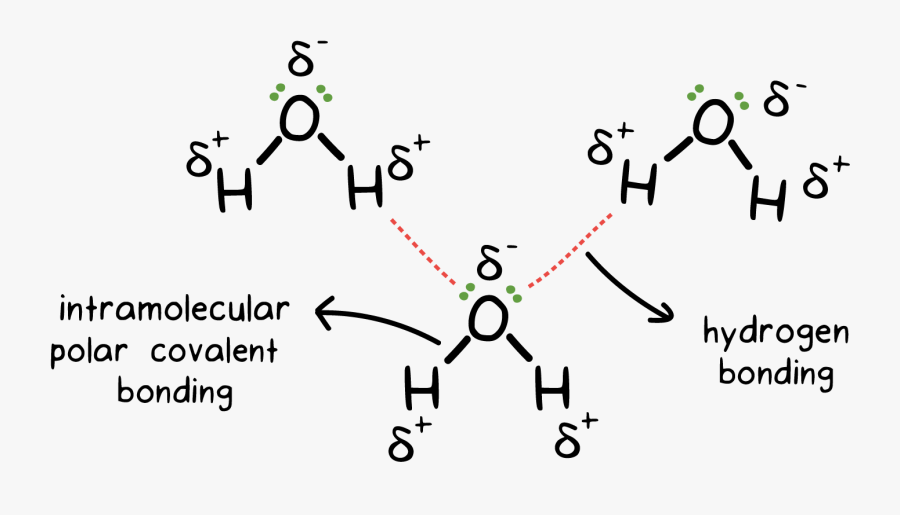
H2o Drawing Chemical Bond Intermolecular Hydrogen Bonding In Water
This Particular Bond Length Represents A Balance Between Several Forces:
The Number Of Lone Pairs On The O Or N.
This Video Shows Three Examples Of Drawing For The Formation Of Hydrogen Bond.
Next, Let's Think About The Carbon Hydrogen Bonds.
Related Post: