How To Draw Dipole
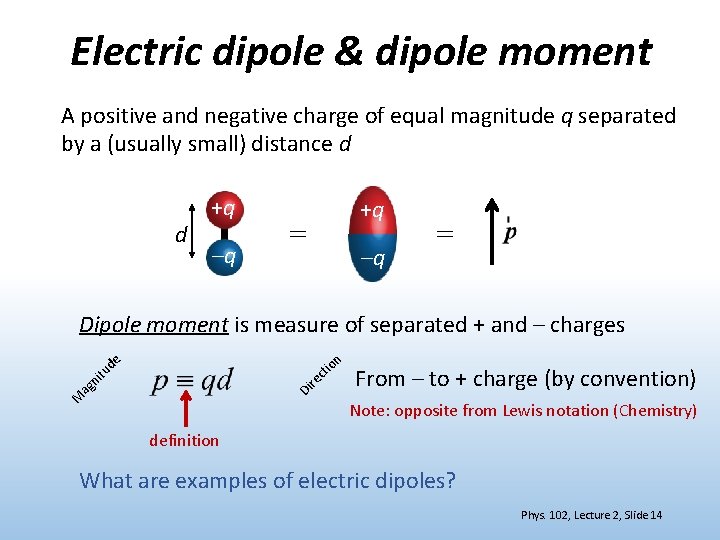
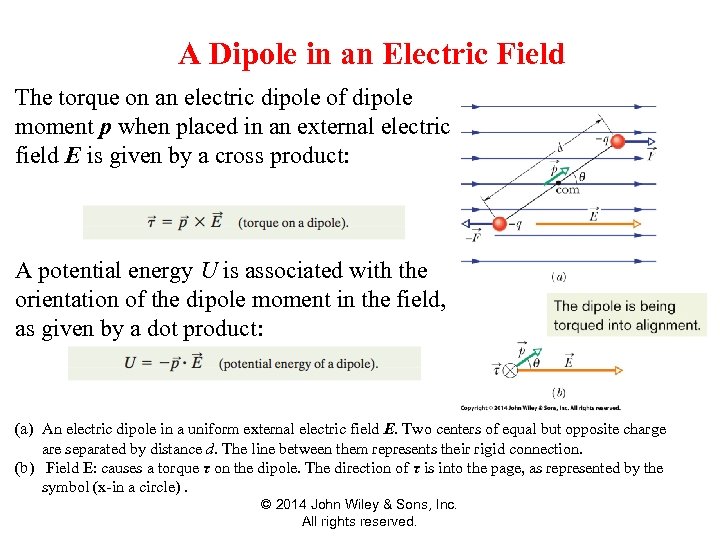
How To Draw Dipole - Intramolecular forces are the forces that hold atoms together within a molecule. After completing this section, you should be able to. This is why in water the dipole arrows are drawn going from hydrogen (low electronegativity) towards oxygen (higher electronegativity). From in between the hydrogen atoms to the oxygen atom. Web dipole arrows are drawn on lewis structures and point towards the more electronegative atom, since they pull electrons towards them. Web this chemistry video tutorial provides a basic introduction into bond polarity, electronegativity, and the dipole moment of a bond. So now we can define the two forces: Δpep = qδv = −qeδr = −qed 2cos θ (11.6.2) (11.6.2) δ p e p = q δ v = − q e δ r = − q e d 2 cos. Just place a substance between charged plates (figure \(\pageindex{2}\)); The bond dipole moment that arises in a chemical bond between two atoms of different electronegativities can be expressed as follows: So now we can define the two forces: The net dipole is the measurable, which is called the dipole. After completing this section, you should be able to. Just place a substance between charged plates (figure \(\pageindex{2}\)); Web now that we understand how to draw dot structures and we know how to predict the shapes of molecules, let's use those. For one bond, the bond dipole moment is determined by the difference in electronegativity between the two atoms. Just place a substance between charged plates (figure \(\pageindex{2}\)); For a positive charge in the dipole the change in potential energy is then: We start by looking at a water molecule: The net dipole is the measurable, which is called the dipole. Web mathematically, dipole moment (µ) = charge (q) * distance of separation (r) it is measured in debye units denoted by ‘d’. Intramolecular forces are the forces that hold atoms together within a molecule. The bond dipole moment that arises in a chemical bond between two atoms of different electronegativities can be expressed as follows: The difference in electronegativity can. The former is termed an intramolecular attraction while the latter is termed an intermolecular attraction. Determine the direction of the magnetic field around the dipole based on the location of the poles, by the flow of the current which. Web a dipole moment measures a separation of charge. When looking at the bond, you compare the two elements' electronegativity. Web. Web it is relatively easy to measure dipole moments: Web mathematically, dipole moment (µ) = charge (q) * distance of separation (r) it is measured in debye units denoted by ‘d’. Web this organic chemistry video explains how to determine if a molecule is polar and has net dipole moment. Web this chemistry video tutorial provides a basic introduction into. Using the cross bow arrow shown below we can show that it has a net dipole. Δpep = qδv = −qeδr = −qed 2cos θ (11.6.2) (11.6.2) δ p e p = q δ v = − q e δ r = − q e d 2 cos. Let's draw the dipoles for all the bonds in carbon. Web mathematically,. Web if a dipole does exist, use a dipole arrow to indicate the direction of the molecular dipole. Using the cross bow arrow shown below we can show that it has a net dipole. Just place a substance between charged plates (figure \(\pageindex{2}\)); We start by looking at a water molecule: For a molecule, the overall dipole moment is determined. It explains how to indicate the polarity of a bond and of a. Web the dipole moment of a molecule and its overall polarity depends on the magnitude and direction of individual polar bonds and their dipole moments. Web this chemistry video tutorial provides a basic introduction into bond polarity, electronegativity, and the dipole moment of a bond. Web dipole. Web dipole arrows are drawn on lewis structures and point towards the more electronegative atom, since they pull electrons towards them. The former is termed an intramolecular attraction while the latter is termed an intermolecular attraction. When this occurs, the partially negative portion of one of the polar molecules is attracted to the partially positive portion of the second polar. Polar molecules increase the charge stored on the plates, and the dipole moment can be obtained (i.e., via the capacitance of. For a molecule, the overall dipole moment is determined by both the individual bond moments and how these dipoles are arranged in the molecular structure. Determine the direction of the magnetic field around the dipole based on the location. The product of the charge and distance between them is called the dipole moment. Just place a substance between charged plates (figure \(\pageindex{2}\)); The bond dipole moment that arises in a chemical bond between two atoms of different electronegativities can be expressed as follows: Web a dipole moment measures a separation of charge. Dipole moment represents the strength of the dipole. 67 views 6 years ago chemistry guided notes. Δpep = qδv = −qeδr = −qed 2cos θ (11.6.2) (11.6.2) δ p e p = q δ v = − q e δ r = − q e d 2 cos. Intramolecular forces are the forces that hold atoms together within a molecule. Young ( chemistryonline.com) via source content that was edited to the style and standards of. Determine the direction of the magnetic field around the dipole based on the location of the poles, by the flow of the current which. Web i believe that within a molecule, you draw dipoles for each bond. When looking at the bond, you compare the two elements' electronegativity. Using the cross bow arrow shown below we can show that it has a net dipole. From in between the hydrogen atoms to the oxygen atom. Web it is relatively easy to measure dipole moments: Web draw the dipole arrow towards the more electronegative atom.How To Draw Overall Dipole Moment DRAWINGS OF LOVE
How To Draw Electric Dipole Moment DRAWINGS OF LOVE

Molecular Polarity Molecular Structure ppt video online download

Molecular Dipole The Overall Polarity of the Molecule Chemistry Steps
[Solved] How do I draw dipole image that has the net dipole vectors and
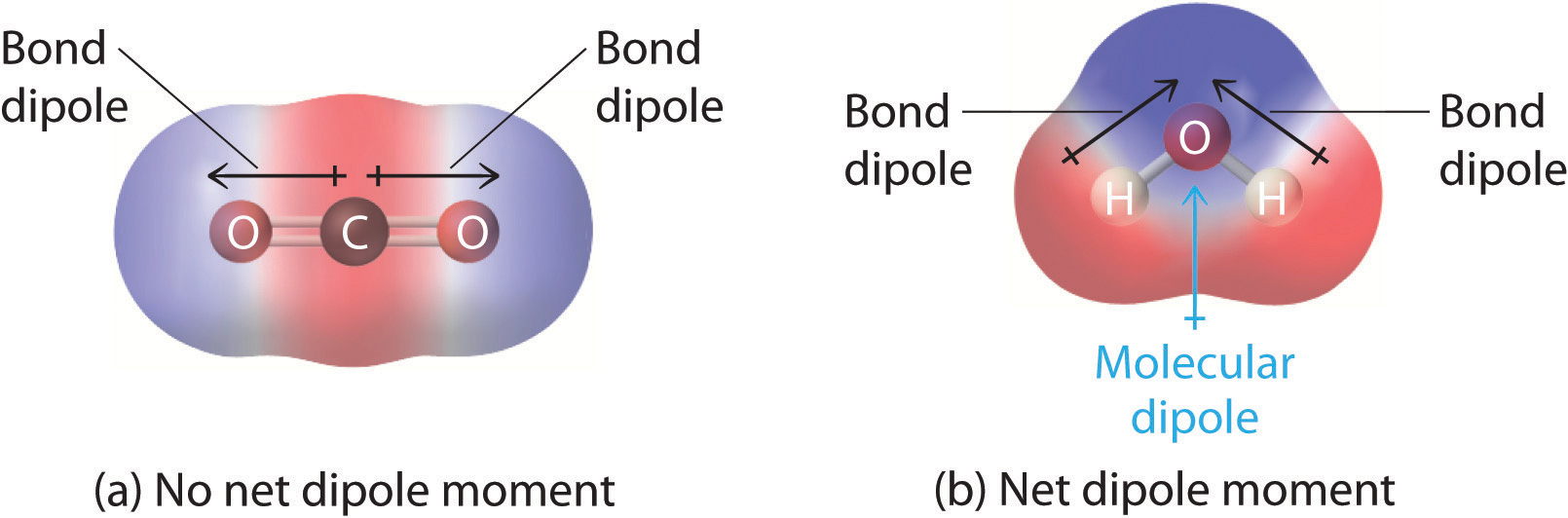
2.2 Polar Covalent Bonds Dipole Moments Chemistry LibreTexts

How To Draw Overall Dipole Moment DRAWINGS OF LOVE
How To Draw Electric Dipole Moment DRAWINGS OF LOVE

Dipole Dipole Forces of Attraction Intermolecular Forces YouTube

Bond Polarity, Electronegativity and Dipole Moment Chemistry Practice
Identify The Type Of Dipole Present.
Explain How Dipole Moments Depend On Both Molecular Shape And Bond Polarity.
We Start By Looking At A Water Molecule:
The Former Is Termed An Intramolecular Attraction While The Latter Is Termed An Intermolecular Attraction.
Related Post: