How To Draw Covalent Compounds
How To Draw Covalent Compounds - Accompanies google classroom hw assigned 4/28, due 4/30. Web in this video, we will go through how to draw lewis structures for covalent compound in five easy steps using carbon dioxide, co2, as an example. Web how to draw covalent bonding molecules. In this video you’ll learn how to draw lewis dot structures for covalent compounds. Consider h and o atoms: Web representing a covalent bond using lewis structures. Therefore, a lewis structure must be drawn for a covalent molecule before its chemical formula can be determined. Using lewis structures, we can represent this as follows: Web draw lewis structures for covalent compounds. 234k views 3 years ago. Examples for drawing lewis structures for covalent bonds. Draw lewis structures for covalent compounds. Web covalent bonds are produced when unpaired electrons found within two atoms interact to form a shared pair of electrons. Web draw lewis structures for covalent compounds. 234k views 3 years ago. The diagram is also called a lewis dot diagram, lewis dot formula, or electron dot diagram. Web here are the steps to draw a lewis structure. Rules for writing covalent formulas; Atoms share the same number of pairs needed to fill their valence shell, usually with eight. Exercise \(\pageindex{1}\) rules for drawing covalent lewis structures; Draw lewis structures for covalent compounds. Accompanies google classroom hw assigned 4/28, due 4/30. Molecules are the simplest unit of a covalent compound, and molecules can be represented in many different ways. The h and o atoms can share an electron to form a covalent bond: Each atom contributes one electron to each shared pair, and effectively gains an additional. 292k views 3 years ago new ap & general chemistry video playlist. Web covalent bonds are produced when unpaired electrons found within two atoms interact to form a shared pair of electrons. For example, consider ccl4 and nf3 as drawn below: Web as covalent bonds are formed by sharing of electrons between atoms, we draw overlapping circles to show the. Lewis structures are representations of molecules that include not only what atoms are present in the molecule but also how the atoms are connected. Consider h and o atoms: For example, consider ccl4 and nf3 as drawn below: An atom that shares one or more of its electrons will complete its outer shell. The following procedure can be used to. Use lewis electron dot diagrams to illustrate the covalent bond formation in cl 2. 234k views 3 years ago. Covalent bonds form when two or more nonmetals combine. The following procedure can be used to construct lewis electron structures for more complex molecules and ions. Using lewis structures, we can represent this as follows: Compounds can be classified as ionic or covalent. The diagram is also called a lewis dot diagram, lewis dot formula, or electron dot diagram. Web the albert team. The following procedure can be used to construct lewis electron structures for simple molecules. How to draw a lewis structure. Web in this video, we will go through how to draw lewis structures for covalent compound in five easy steps using carbon dioxide, co2, as an example. The electrons involved are in the outer shells of the atoms. Exercise \(\pageindex{1}\) rules for drawing covalent lewis structures; Use lewis electron dot diagrams to illustrate the covalent bond formation in cl 2.. Each atom contributes one electron to each shared pair, and effectively gains an additional electron from the shared pair. 234k views 3 years ago. More than two atoms can participate in covalent bonding, although any given covalent bond will be between two atoms only. A lewis structure is a diagram that shows the chemical bonds between atoms in a molecule. Rules for writing covalent formulas; Use lewis electron dot diagrams to illustrate the covalent bond formation in cl 2. Compounds can be classified as ionic or covalent. 4.7k views 7 years ago 1d: Molecules are the simplest unit of a covalent compound, and molecules can be represented in many different ways. Web dr bp explains how to draw lewis dot structures of covalent compounds. More than two atoms can participate in covalent bonding, although any given covalent bond will be between two atoms only. Web covalent bonds are produced when unpaired electrons found within two atoms interact to form a shared pair of electrons. 1.31 explain the formation of simple molecular, covalent substances, using dot and cross diagrams, including: Atoms are the smallest units of matter that still retain the fundamental chemical properties of an element. Examples for drawing lewis structures for covalent bonds. Atoms share the same number of pairs needed to fill their valence shell, usually with eight. In this video you’ll learn how to draw lewis dot structures for covalent compounds. Using lewis structures, we can represent this as follows: Each atom contributes one electron to each shared pair, and effectively gains an additional electron from the shared pair. For covalent bonding, we often want to draw how the atoms share electrons in the molecule. Web representing a covalent bond using lewis structures. The example is for the nitrate ion. Web how to draw covalent bonding molecules. Lewis structures are representations of molecules that include not only what atoms are present in the molecule but also how the atoms are connected. An atom that shares one or more of its electrons will complete its outer shell.
How to Draw Covalent Compounds YouTube

How is a covalent bond formed

Covalent Bonding Diagram

CHEMISTRY 101 Draw Lewis dot structures for covalent compounds YouTube

How to Draw Lewis Dot Structure of Covalent Compounds Chemical

How to Draw Covalent Compounds as Lewis Structures Chemistry for
/some-examples-of-covalent-compounds-603981_final21-a3faebbe543e404fb951d2e789031f56.jpg)
Examples of Covalent Bonds and Compounds

Chemical Bonds, Ionic, Covalent and Metallic AQA C2 revisechemistry.uk
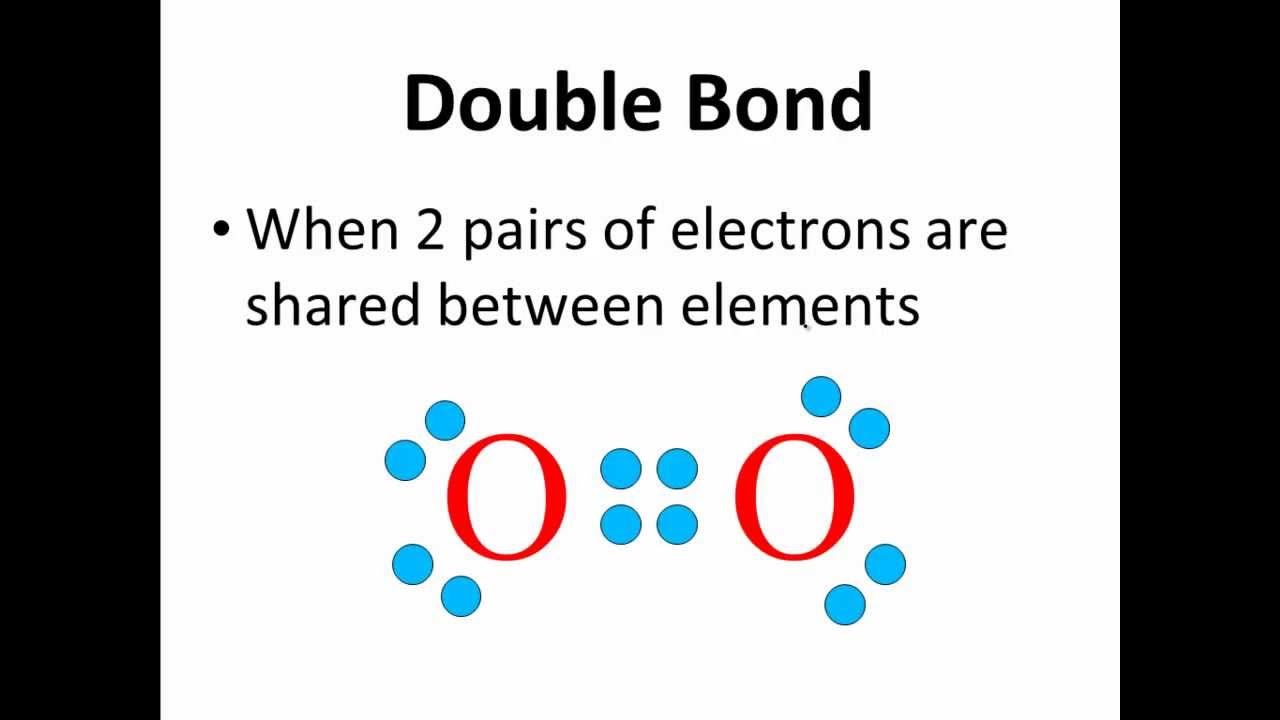
Lewis Dot Structures for Covalent Compounds Part 1 CLEAR & SIMPLE
Covalent Compounds
One Line Is A Single Bond With 2 Bonding Electrons, Two Lines Is A Double Bond With 4 Bonding Electrons, And Three Lines Is A Triple Bond With 6 Bonding Electrons.
Each Line Represents Two Electrons That Are Being Shared.
Using Lewis Structures To Show Valence Electrons.
For Example, Consider Ccl4 And Nf3 As Drawn Below:
Related Post: