How To Draw An Electron Dot Diagram
How To Draw An Electron Dot Diagram - Shared pairs of electrons are drawn as lines between atoms, while lone pairs of electrons are drawn as dots next to atoms. The video covers the basic lewis structures you'll see in an introductory chemistry class. How to draw a lewis structure. Lewis dot structure of atoms comprising ammonia and the dot structure of the molecule. Using lewis structures to show valence electrons. The first thing we would need to do is to find the total number of valence electrons. Atoms and the periodic table. Web here's some of the guidelines for drawing dot structures. A simplified way to show valence electrons. Since electrons repel each other, the dots for a given atom are distributed evenly around the symbol before they are paired. Web to draw the lewis structure of an atom, write the symbol of the atom and draw dots around it to represent the valence electrons. Only give reasonable results for covalent compounds and polyatomic ions of the main group (s and p block) elements, can not predict the structure of ionic compounds, Web finally, you'll understand all those weird pictures. In almost all cases, chemical bonds are formed by interactions of valence electrons in atoms. Web lewis electron dot diagrams use dots to represent valence electrons around an atomic symbol. Using the periodic table to draw lewis dot structures. Web here are the steps to draw a lewis structure. A lewis electron dot formula comprises one dot for every valence. Those are lewis dot structures. In almost all cases, chemical bonds are formed by interactions of valence electrons in atoms. Draw a lewis electron dot diagram for an atom or a monatomic ion. Only give reasonable results for covalent compounds and polyatomic ions of the main group (s and p block) elements, can not predict the structure of ionic compounds,. The diagram is also called a lewis dot diagram, lewis dot formula, or electron dot diagram. Only give reasonable results for covalent compounds and polyatomic ions of the main group (s and p block) elements, can not predict the structure of ionic compounds, Public domain) how do we show electrons in atoms? Using lewis structures to show valence electrons. Remember. By kirsty patterson 6 september 2021. The reason for learning to draw lewis structures is to predict the number and type of bonds that may be formed around an atom. The example is for the nitrate ion. Only give reasonable results for covalent compounds and polyatomic ions of the main group (s and p block) elements, can not predict the. The reason for learning to draw lewis structures is to predict the number and type of bonds that may be formed around an atom. Those are lewis dot structures. Web to draw the lewis structure of an atom, write the symbol of the atom and draw dots around it to represent the valence electrons. To facilitate our understanding of how. Web finally, you'll understand all those weird pictures of molecules with the letters and the lines and the dots! Web you can use a drawing or use small beads or objects to represent electrons and work out the electron arrangement. In almost all cases, chemical bonds are formed by interactions of valence electrons in atoms. Explain the meaning of an. Those are lewis dot structures. In almost all cases, chemical bonds are formed by interactions of valence electrons in atoms. The example is for the nitrate ion. Lewis electron dot diagrams for ions have fewer (for cations) or more (for anions) dots than the corresponding atom. Diagrams contain a lot of useful information in a compact format. Web you can use a drawing or use small beads or objects to represent electrons and work out the electron arrangement. The lewis dot structure for formaldehyde. Use a different color or shape to represent the electrons of each atom. The diagram is also called a lewis dot diagram, lewis dot formula, or electron dot diagram. A lewis structure is. A simplified way to show valence electrons. Web electron dot diagrams are diagrams in which the valence electrons of an atom are shown as dots distributed around the element’s symbol. The example is for the nitrate ion. To facilitate our understanding of how valence electrons interact, a simple way of representing those valence electrons would be useful. How to draw. Remember that lewis dot structures. A simplified way to show valence electrons. Since electrons repel each other, the dots for a given atom are distributed evenly around the symbol before they are paired. Web how to draw electron dot structures? A lewis electron dot formula comprises one dot for every valence electron and an element’s symbol. Diagrams contain a lot of useful information in a compact format. Draw a lewis electron dot diagram for an atom or a monatomic ion. Web finally, you'll understand all those weird pictures of molecules with the letters and the lines and the dots! Draw a lewis electron dot symbol for a given atom. Atoms and the periodic table. In almost all cases, chemical bonds are formed by interactions of valence electrons in atoms. The lewis dot structure for formaldehyde. In almost all cases, chemical bonds are formed by interactions of valence electrons in atoms. Web these diagrams are helpful because they allow us to show how atoms are connected, and when coupled with valence shell electron repulsion theory (vsepr), we can use lewis structures to predict the shape of the molecule. By kirsty patterson 6 september 2021. Stages to articulate the electron dot formula are stated beneath.
Drawing Lewis Dot Structures for Chemistry dummies

3 Ways to Draw Lewis Dot Structures wikiHow
/Lewis-dot-structure-58e5390f3df78c5162b4c3db.jpg)
How to Draw a Lewis Structure
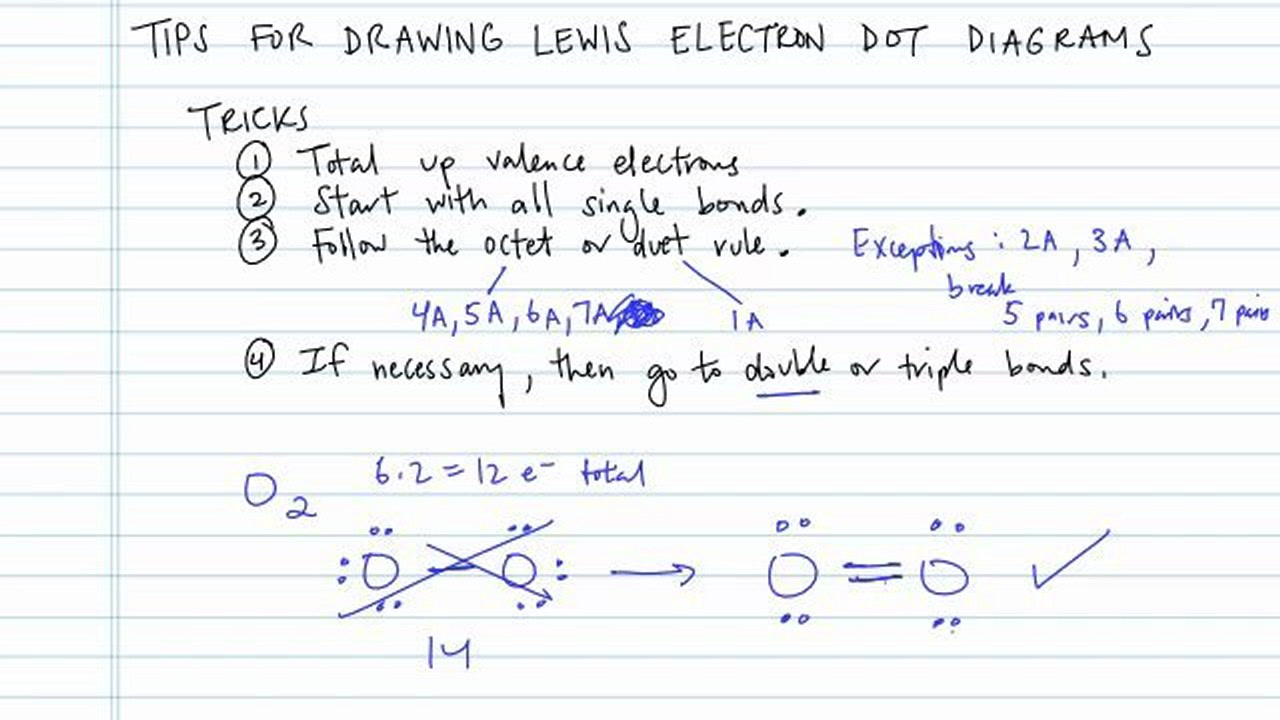
How To Draw Electron Dot Diagrams Elementchampionship Jeffcoocctax

3 Ways to Draw Lewis Dot Structures wikiHow

BohrRutherford Diagrams & Lewis Dot Diagrams Eve Wongworakul

How to Draw a Lewis Structure

Molecular Modeling Digital and Analog General Chemistry Lab News

Lewis Electron Dot Structure Calculator

How To Draw Electron Dot Diagrams Elementchampionship Jeffcoocctax
In Almost All Cases, Chemical Bonds Are Formed By Interactions Of Valence Electrons In Atoms.
Web To Draw The Lewis Structure Of An Atom, Write The Symbol Of The Atom And Draw Dots Around It To Represent The Valence Electrons.
To Facilitate Our Understanding Of How Valence Electrons Interact, A Simple Way Of Representing Those Valence Electrons Would Be Useful.
Draw A Lewis Electron Dot Diagram For An Atom Or A Monatomic Ion.
Related Post: