H20 Drawing
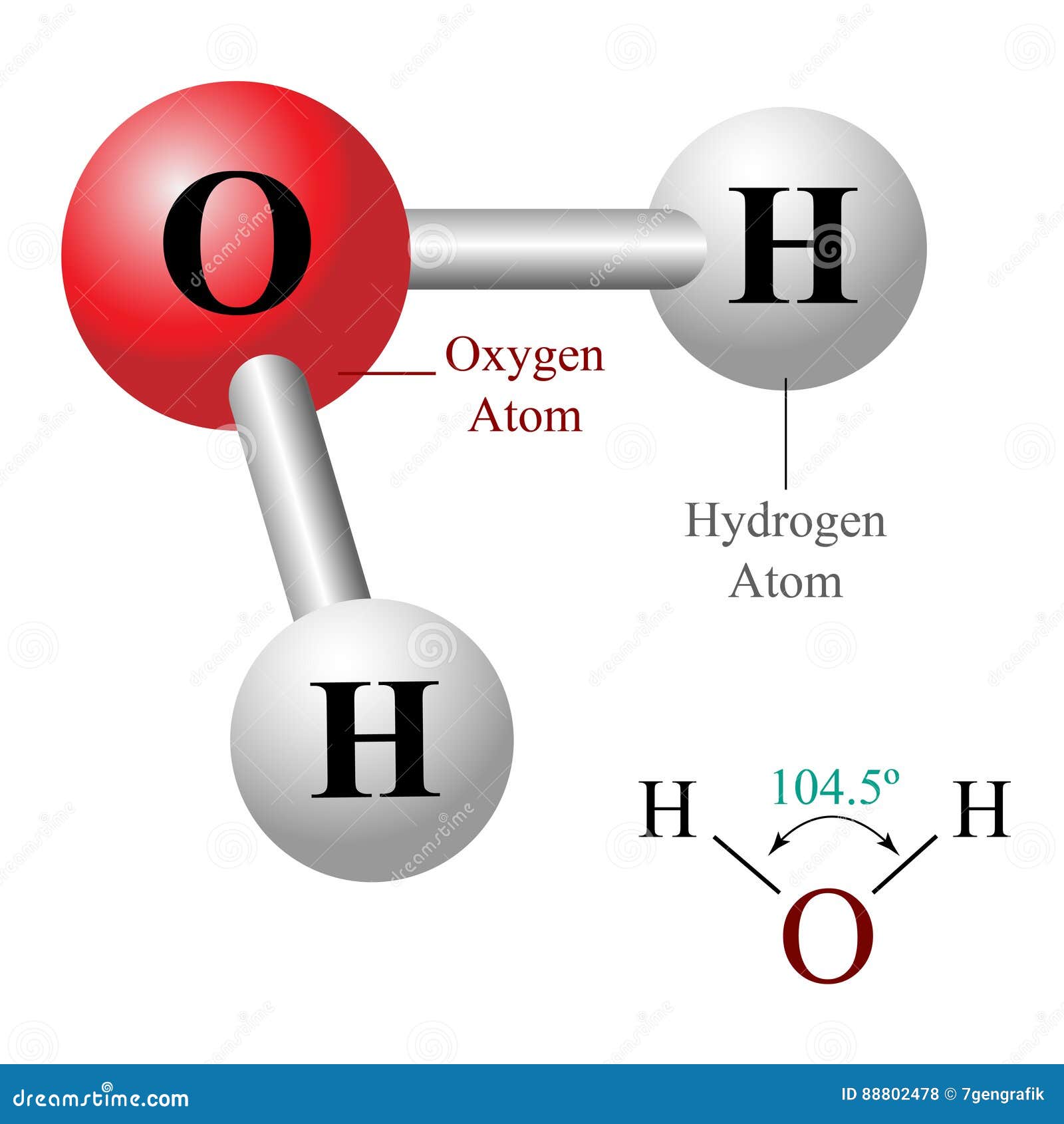
H20 Drawing - Count the total valence electrons. Web 6 steps to draw the lewis structure of h2o. On this picture you can see tetrahedral shapes of water, ammonia and methane. For the h2o structure use the periodic table to find the total number of valence electrons for the. In order to draw the lewis structure. Remember that hydrogen only needs two electrons to have a full outer. Web a water molecule consists of two hydrogen atoms bonded to an oxygen atom, and its overall structure is bent. I also go over hybridization, shape and bond angle. Web here, we need to understand how the lewis structure is drawn for the h2o molecule: Here, the given molecule is h2o (water). For h2o, there are 2 valence electrons for. Web here are the steps to draw the h2o lewis structure: Count the total valence electrons. In the periodic table, hydrogen lies in group 1, and. 22k views 1 year ago. Count the total valence electrons. Here, the given molecule is h2o (water). You can find out more about the vsepr shape of water and the symmetry of water. Valence electrons are the electrons in the outermost shell of an atom. Find the point group of the molecule. Web a water molecule consists of two hydrogen atoms bonded to an oxygen atom, and its overall structure is bent. Remember that hydrogen only needs two electrons to have a full outer. Web here, we need to understand how the lewis structure is drawn for the h2o molecule: Determine the total number of valence electrons for all the atoms in. Web 6 steps to draw the lewis structure of h2o. Determine the total number of electrons in the valence shells of hydrogen and oxygen. Web lewis structure of water molecule contains two single bonds. Valence electrons are the electrons in the outermost shell of an atom. Water has tetrahedral shape, or to be more precise, bent shape. Web a water molecule consists of two hydrogen atoms bonded to an oxygen atom, and its overall structure is bent. Web #1 draw a rough skeleton structure. Web here, we need to understand how the lewis structure is drawn for the h2o molecule: Number of total valence electrons of oxygen and hydrogen atoms are used to draw lewis structure. 22k. Remember that hydrogen only needs two electrons to have a full outer. This is because the oxygen atom, in addition to forming bonds with the. Determine the total number of valence electrons for all the atoms in the molecule. H2o molecular geometry, lewis structure, shape and bond angles. In this article, we will delve. Web 6 steps to draw the lewis structure of h2o. Web #1 draw a rough skeleton structure. Look for the total valence electrons: How to draw the dot structure for h2o | chemical bonding | success in chemistry. Web because of the two lone pairs, h 2 o will have a bent molecular geometry and it will be a polar. I also go over hybridization, shape and bond angle. Construct salcs and the molecular orbital diagram for h 2 o. Look for the total valence electrons: How to draw the dot structure for h2o | chemical bonding | success in chemistry. Web follow these steps to draw the lewis structure of h2o: For the h2o structure use the periodic table to find the total number of valence electrons for the. Look for the total valence electrons: Drawing the lewis structure for water. Let's do the lewis structure for. Web lewis structure of water molecule contains two single bonds. Web lewis structure of water molecule contains two single bonds. Count the total valence electrons. Number of total valence electrons of oxygen and hydrogen atoms are used to draw lewis structure. For the h2o structure use the periodic table to find the total number of valence electrons for the. In this article, we will delve. This is the lewis dot structure for h2o. H2o molecular geometry, lewis structure, shape and bond angles. Construct salcs and the molecular orbital diagram for h 2 o. It is eight to form a single h2o molecule. Find the point group of the molecule. For h2o, there are 2 valence electrons for. You could alternatively also draw the structure by including two dots for every bond. Web follow these steps to draw the lewis structure of h2o: For the h2o structure use the periodic table to find the total number of valence electrons for the. This is because the oxygen atom, in addition to forming bonds with the. Determine the total number of valence electrons for all the atoms in the molecule. Look for the total valence electrons: Web because of the two lone pairs, h 2 o will have a bent molecular geometry and it will be a polar molecule. Calculate the total number of valence electrons. Web a water molecule consists of two hydrogen atoms bonded to an oxygen atom, and its overall structure is bent. Count the total valence electrons.
Download H2O Water Molecule RoyaltyFree Stock Illustration Image Pixabay
Chemical structure water molecule h2o Banque d'images vectorielles Alamy
Chemistry model of molecule water H2O scientific elements. Integrated

H2O Lewis Structure, Molecular Geometry, and Hybridization

H2O Lewis Structure, Molecular Geometry, and Hybridization

H2O. Molécule d'eau modèle, formule chimique, ballandstick modèle
Chemistry model of molecule water H2O scientific elements. Integrated
H2O, Water Molecule Illustration Stock Vector Illustration of core

How it Works Maximum H20

H2o water molecule model chemical formula Vector Image
Web I Quickly Take You Through How To Draw The Lewis Structure Of Water, H2O.
In This Article, We Will Delve.
Web Here, We Need To Understand How The Lewis Structure Is Drawn For The H2O Molecule:
For The H2O Structure Use The Periodic Table To Find The Total Number Of Valence Electrons For The.
Related Post: