Electron Drawing
Electron Drawing - The first electron has the same four quantum numbers as the hydrogen atom electron ( n = 1, l = 0, ml = 0, ms = +12 m s = + 1 2 ). This is sometimes called the bohr, or the ‘solar system’, model. Web a lewis diagram shows how the valence electrons are distributed around the atoms in a molecule. Web an electron configuration diagram is a model that depicts the position of electrons as they orbit the nucleus of an atom. Web electron configurations describe where electrons are located around the nucleus of an atom. Web the electron configuration and the orbital diagram are: The helium atom contains two protons and two electrons. Web once you’ve remembered and drawn the table, just follow the arrow (starting from the top) until the subscripts add up to the total number of electrons in your atom. Web a lewis electron dot diagram (or electron dot diagram, or a lewis diagram, or a lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. 1.1m views 9 years ago chemistry: Shared pairs of electrons are drawn as lines between atoms, while lone pairs of electrons are drawn as dots next to atoms. Web a lewis electron dot diagram (or electron dot diagram, or a lewis diagram, or a lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. The. This is sometimes called the bohr, or the ‘solar system’, model. Electrons are represented by dots or crosses and are positioned in energy levels, or ‘shells’, around the central nucleus. The helium atom contains two protons and two electrons. Web a lewis diagram shows how the valence electrons are distributed around the atoms in a molecule. For example, the electron. The number of dots equals the number of valence electrons in the atom. The helium atom contains two protons and two electrons. To understand the basics of. It explains how to write the orbital diagram n. Web an electron configuration diagram is a model that depicts the position of electrons as they orbit the nucleus of an atom. Web an electron configuration diagram is a model that depicts the position of electrons as they orbit the nucleus of an atom. The first electron has the same four quantum numbers as the hydrogen atom electron ( n = 1, l = 0, ml = 0, ms = +12 m s = + 1 2 ). Web this chemistry video. When writing an electron configuration, first write the energy level (the period), then the subshell to be filled and the superscript, which is the number of electrons in that subshell. The first electron has the same four quantum numbers as the hydrogen atom electron ( n = 1, l = 0, ml = 0, ms = +12 m s =. The first electron has the same four quantum numbers as the hydrogen atom electron ( n = 1, l = 0, ml = 0, ms = +12 m s = + 1 2 ). The helium atom contains two protons and two electrons. Web electron configurations describe where electrons are located around the nucleus of an atom. The number of. Following hydrogen is the noble gas helium, which has an atomic number of 2. For example, the electron configuration of lithium, 1 s ²2 s ¹, tells us that lithium has two electrons in the 1 s subshell and one electron in the 2 s subshell. Web this chemistry video tutorial provides a basic introduction into orbital diagrams and electron. This is sometimes called the bohr, or the ‘solar system’, model. Web electron configurations describe where electrons are located around the nucleus of an atom. Web a lewis electron dot diagram (or electron dot diagram, or a lewis diagram, or a lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of. Web the electron configuration and the orbital diagram are: It explains how to write the orbital diagram n. To understand the basics of adding electrons to atomic orbitals. Web electron configurations describe where electrons are located around the nucleus of an atom. 1.1m views 9 years ago chemistry: Web a lewis diagram shows how the valence electrons are distributed around the atoms in a molecule. 1.1m views 9 years ago chemistry: It explains how to write the orbital diagram n. Web electron configurations describe where electrons are located around the nucleus of an atom. Electrons are represented by dots or crosses and are positioned in energy levels, or. Electrons are represented by dots or crosses and are positioned in energy levels, or ‘shells’, around the central nucleus. Following hydrogen is the noble gas helium, which has an atomic number of 2. Web electron configurations describe where electrons are located around the nucleus of an atom. The helium atom contains two protons and two electrons. The first electron has the same four quantum numbers as the hydrogen atom electron ( n = 1, l = 0, ml = 0, ms = +12 m s = + 1 2 ). Web a lewis diagram shows how the valence electrons are distributed around the atoms in a molecule. The total number of electrons is the atomic number, z. Web the electron configuration and the orbital diagram are: Shared pairs of electrons are drawn as lines between atoms, while lone pairs of electrons are drawn as dots next to atoms. For example, the electron configuration of lithium, 1 s ²2 s ¹, tells us that lithium has two electrons in the 1 s subshell and one electron in the 2 s subshell. Web an electron configuration diagram is a model that depicts the position of electrons as they orbit the nucleus of an atom. Web a lewis electron dot diagram (or electron dot diagram, or a lewis diagram, or a lewis structure) is a representation of the valence electrons of an atom that uses dots around the symbol of the element. Web once you’ve remembered and drawn the table, just follow the arrow (starting from the top) until the subscripts add up to the total number of electrons in your atom. To understand the basics of. Electron configuration diagrams | properties of matter | chemistry | fuseschool learn the basics about drawing electron. Web this chemistry video tutorial provides a basic introduction into orbital diagrams and electron configuration.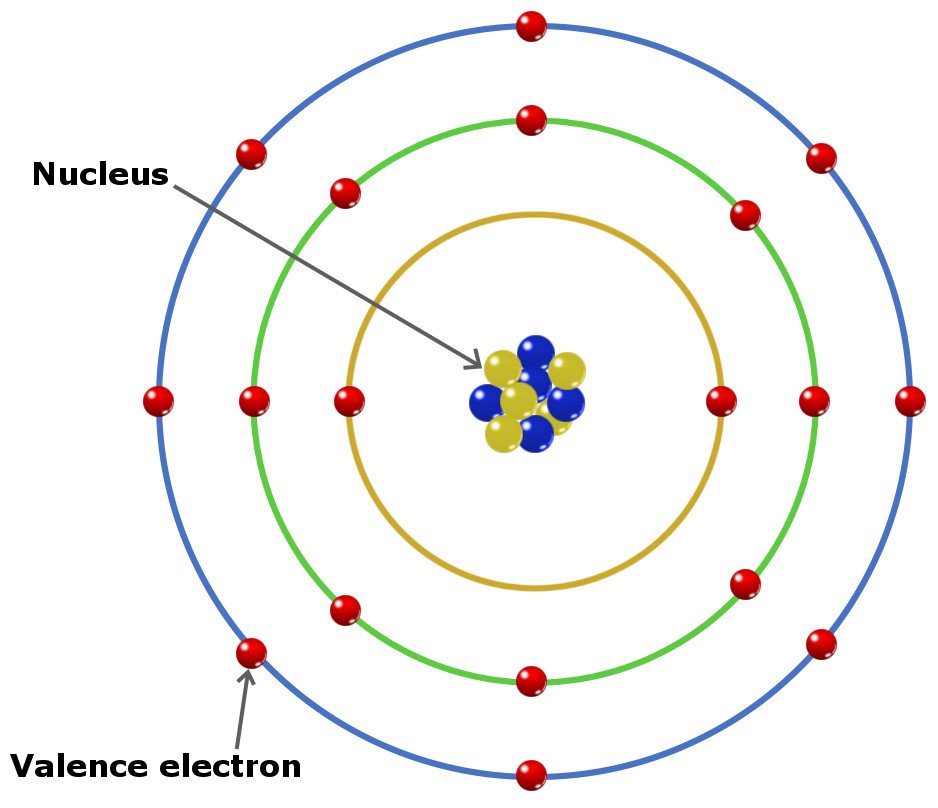
What Are Valence Electrons And How To Find Them? Where Are They Located?
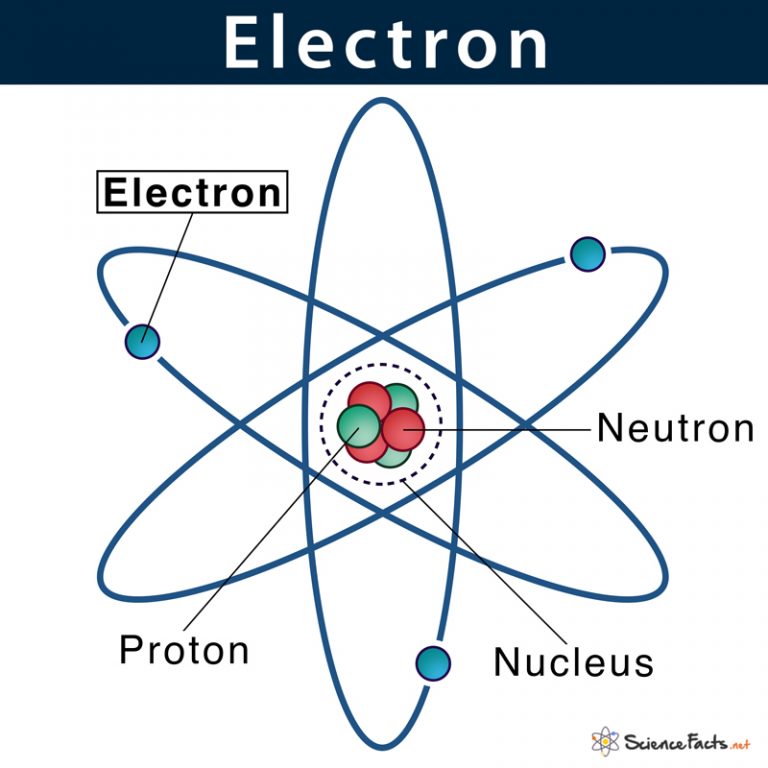
Atomic Nucleus Definition, Structure & Parts with Diagram
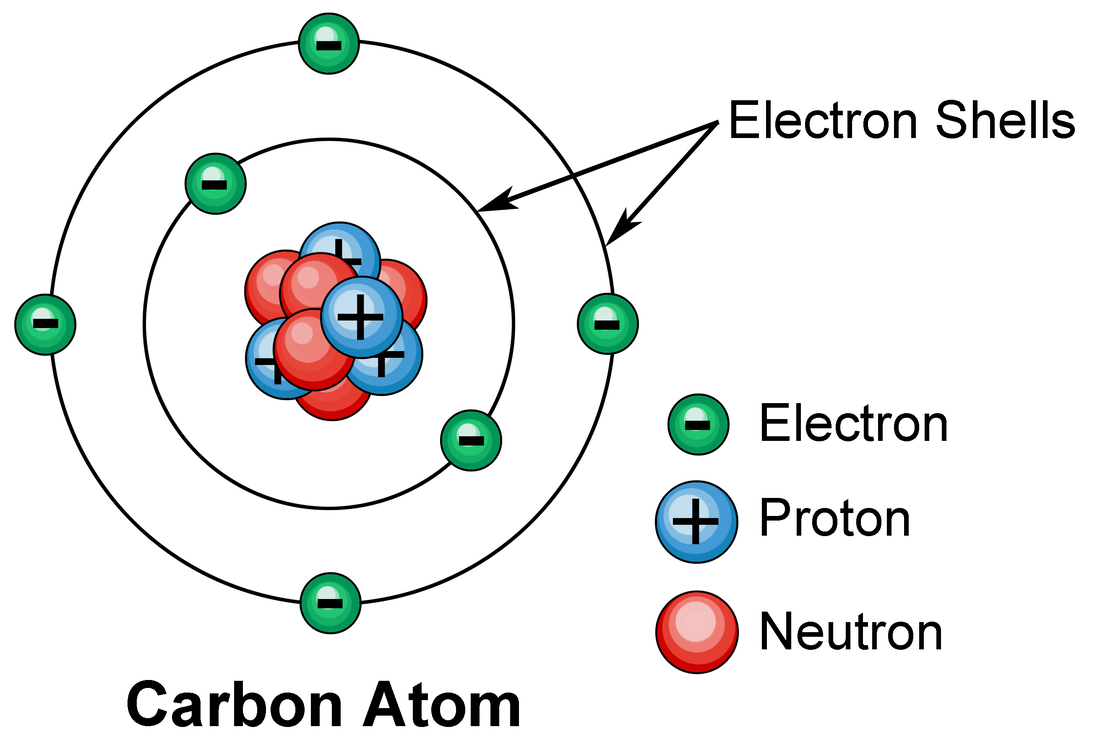
Electronic structure of matter. San Francisco de Paula, Science
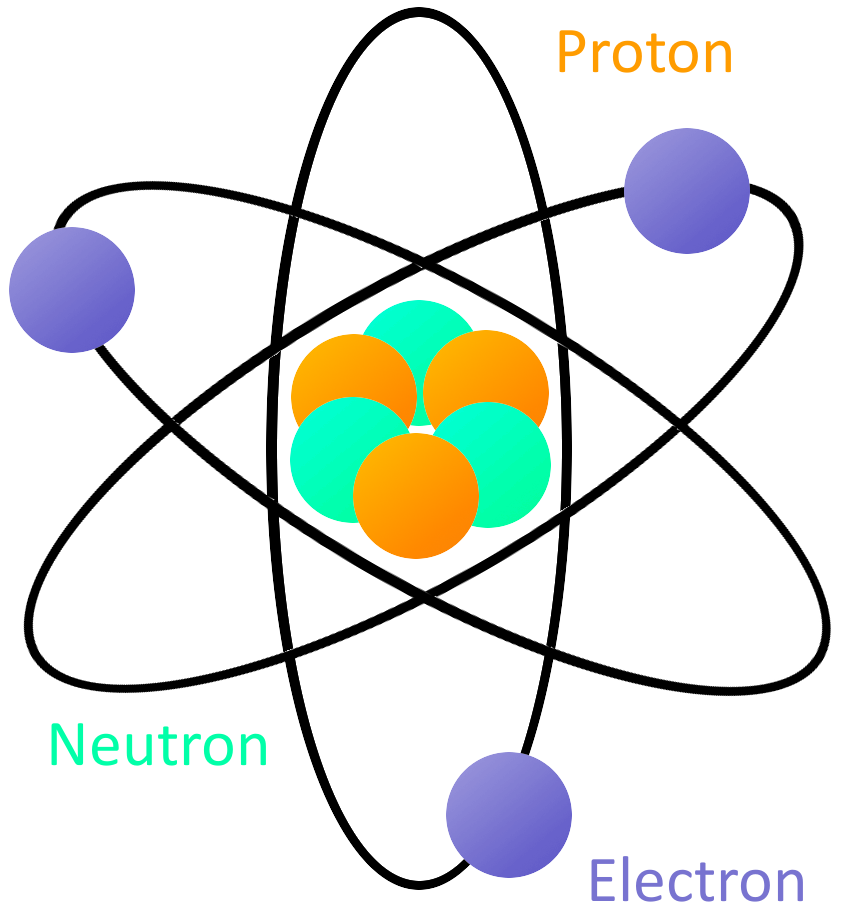
What is Electricity? SparkFun Learn

Atom Drawing
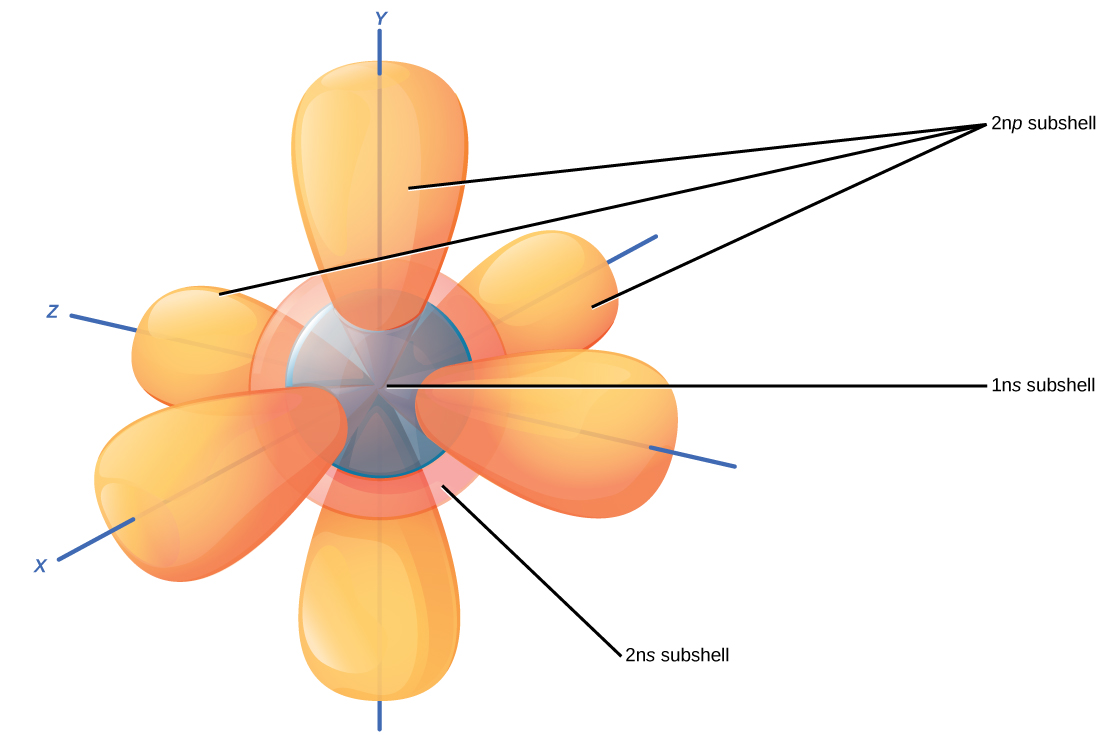
Electrons Biology for Majors I

Atomic Structure Broad Learnings

3.7 Electron Arrangement The Quantum Model Chemistry LibreTexts
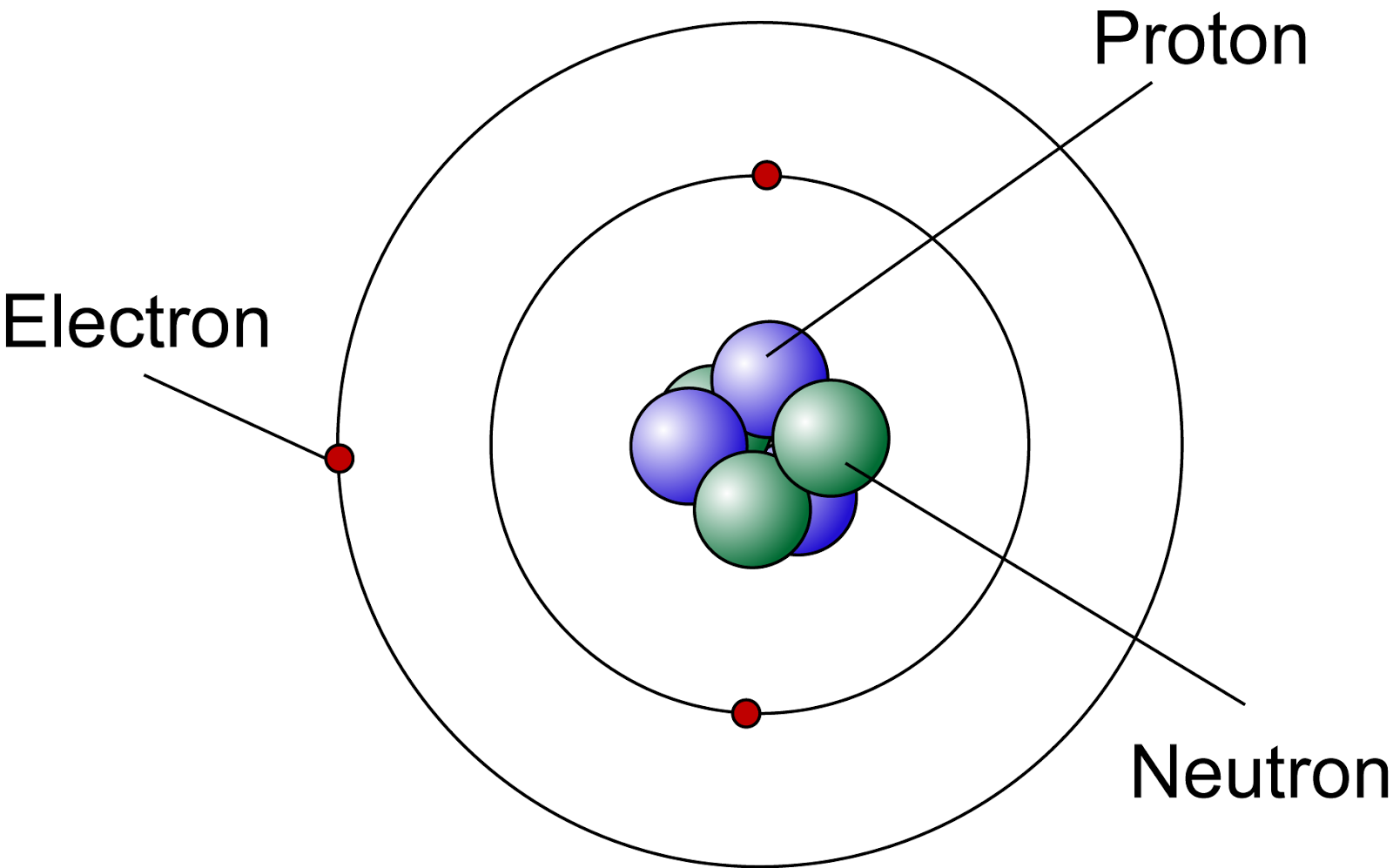
Lets Get Inside An Atom!! The Science Station
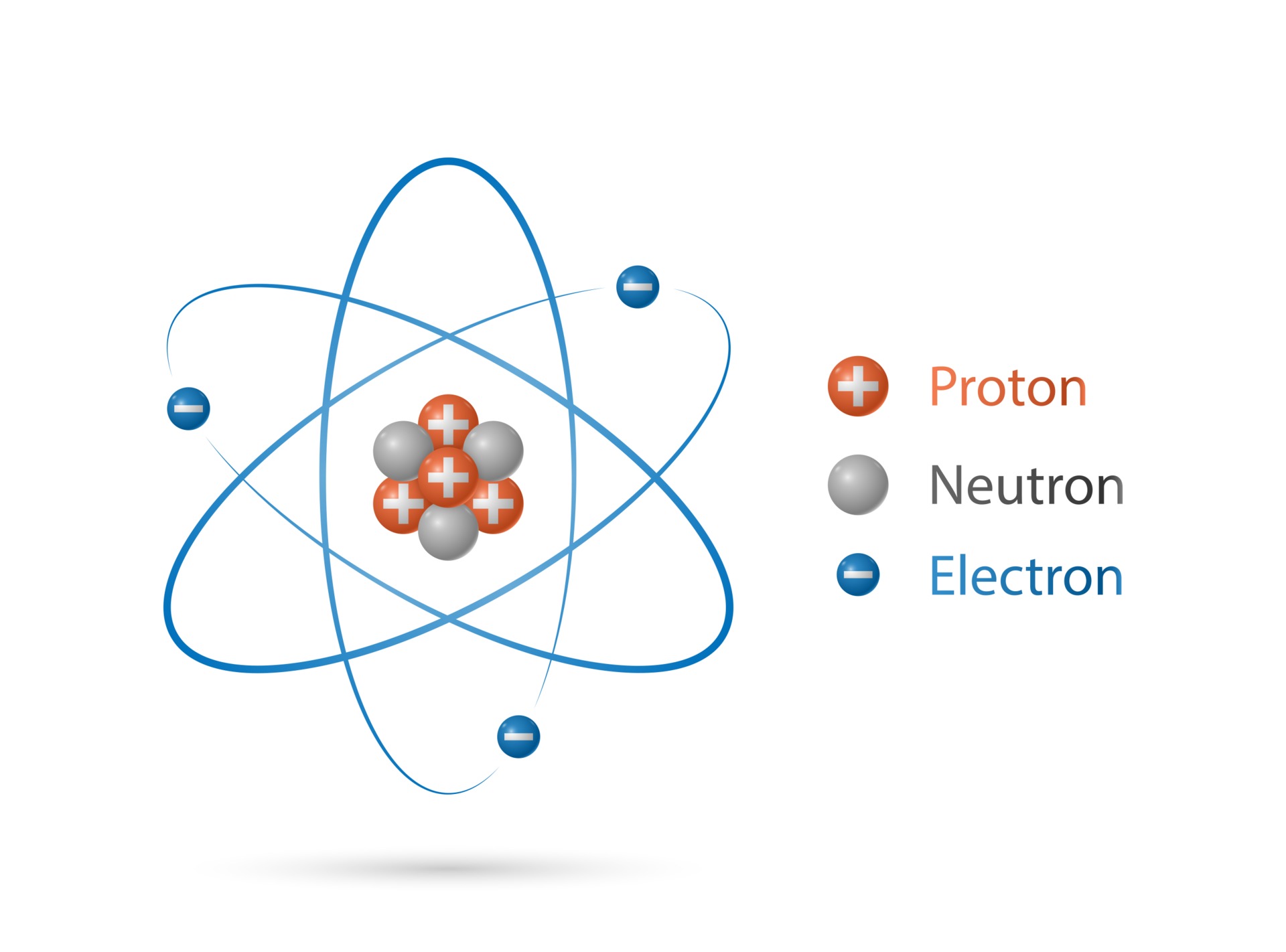
Atomic Structure Vector Art, Icons, and Graphics for Free Download
1.1M Views 9 Years Ago Chemistry:
The Number Of Dots Equals The Number Of Valence Electrons In The Atom.
This Is Sometimes Called The Bohr, Or The ‘Solar System’, Model.
When Writing An Electron Configuration, First Write The Energy Level (The Period), Then The Subshell To Be Filled And The Superscript, Which Is The Number Of Electrons In That Subshell.
Related Post: