Drawing Resonance
Drawing Resonance - Why are they considered different? We can convert one resonance form into another by showing the movement of electrons between bonds and lone pairs (or vice versa). Use the concept of resonance to explain structural features of molecules and ions. Some molecules have two or more chemically equivalent lewis electron structures, called resonance structures. In both examples, the carbon ring has one atom that is bonded to two hydrogens instead of one. Web identifying the correct resonating structure. A charged species is represented in an image given below. Web © 2024 google llc. Resonance is a mental exercise and method within the valence bond theory of bonding that describes the delocalization of electrons within molecules. Then exchange the positions of the multiple bond and the electrons in the p orbital. Use the concept of resonance to explain structural features of molecules and ions. Web a resonance form is another way of drawing a lewis dot structure for a given compound. , aren't the three dot structures the same, just rotated? Use the concept of resonance to explain structural features of molecules and ions. Equivalent lewis structures are called resonance forms. It compares and contrasts two or more possible lewis structures that can represent a particular molecule. How to avoid common mistakes when drawing resonance structures. Web identifying the correct resonating structure. Web draw three resonance forms for the pentadienyl radical, where a radical is a substance that contains a single, unpaired electron in one of its orbitals, denoted by a. Then exchange the positions of the multiple bond and the electrons in the p orbital. It explains how to draw the resonance structures using curved. , aren't the three dot structures the same, just rotated? Web resonance is a mental exercise and method within the valence bond theory of bonding that describes the delocalization of electrons within molecules. Use the. A charged species is represented in an image given below. They are used when there is more than one way to place double bonds and lone pairs on atoms. Equivalent lewis structures are called resonance forms. All right, let's look at an application of the acetate anion here, and the resonance structures that we can draw. Web this general chemistry. Combine the resonance structures by adding (dotted) bonds where other resonance bonds can be formed. Then exchange the positions of the multiple bond and the electrons in the p orbital. , aren't the three dot structures the same, just rotated? 341k views 11 years ago. Resonance exists only when a lewis structure has multiple bonds and an adjacent atom with. Web a resonance form is another way of drawing a lewis dot structure for a given compound. Want to join the conversation? Identify the incorrect resonating structure for the given charged species. All right, let's look at an application of the acetate anion here, and the resonance structures that we can draw. Web draw the resonance structures of molecules or. All resonance structures must be valid lewis structures. It explains how to identify the major resonance contributor as well as the minor. They are used when there is more than one way to place double bonds and lone pairs on atoms. , aren't the three dot structures the same, just rotated? Want to join the conversation? Draw the lewis structure & resonance. It compares and contrasts two or more possible lewis structures that can represent a particular molecule. Web draw the resonance structures of molecules or ions that exhibit delocalization. Use the concept of resonance to explain structural features of molecules and ions. Resonance exists only when a lewis structure has multiple bonds and an adjacent. Some molecules have two or more chemically equivalent lewis electron structures, called resonance structures. ( keep in mind that all the rules applied to lewis structures still apply here!) all resonance structures must have the same atom connectivity and only differ in the electron arrangement. Resonance exists only when a lewis structure has multiple bonds and an adjacent atom with. How to identify the molecules having resonance. Web this organic chemistry video tutorial provides a basic introduction into drawing resonance structures. We just need a graphical tool to do it. Want to join the conversation? Draw the lewis structure & resonance. Web draw the resonance structures of molecules or ions that exhibit delocalization. Then exchange the positions of the multiple bond and the electrons in the p orbital. This real structure (the resonance hybrid) takes its character from the average of all the individual resonance contributors. Combine the resonance structures by adding (dotted) bonds where other resonance bonds can be formed. Equivalent lewis structures are called resonance forms. Web drawing resonance structures and resonance hybrid using example of nitrate anion. Web © 2024 google llc. It explains how to draw the resonance structures using curved. Introducing curved arrows, a tool for showing the movement of electrons between resonance structures. Resonance is a mental exercise and method within the valence bond theory of bonding that describes the delocalization of electrons within molecules. Begin by watching this video: Web resonance is a mental exercise and method within the valence bond theory of bonding that describes the delocalization of electrons within molecules. Web drawing lewis structures: Determine the relative stability of resonance structures using a set of rules. If it has only one lewis structure, it doesn’t have a resonance hybrid. It explains how to identify the major resonance contributor as well as the minor.
Resonance Structures YouTube

drawing & ranking resonance forms YouTube

Rules for Drawing Resonance Structures
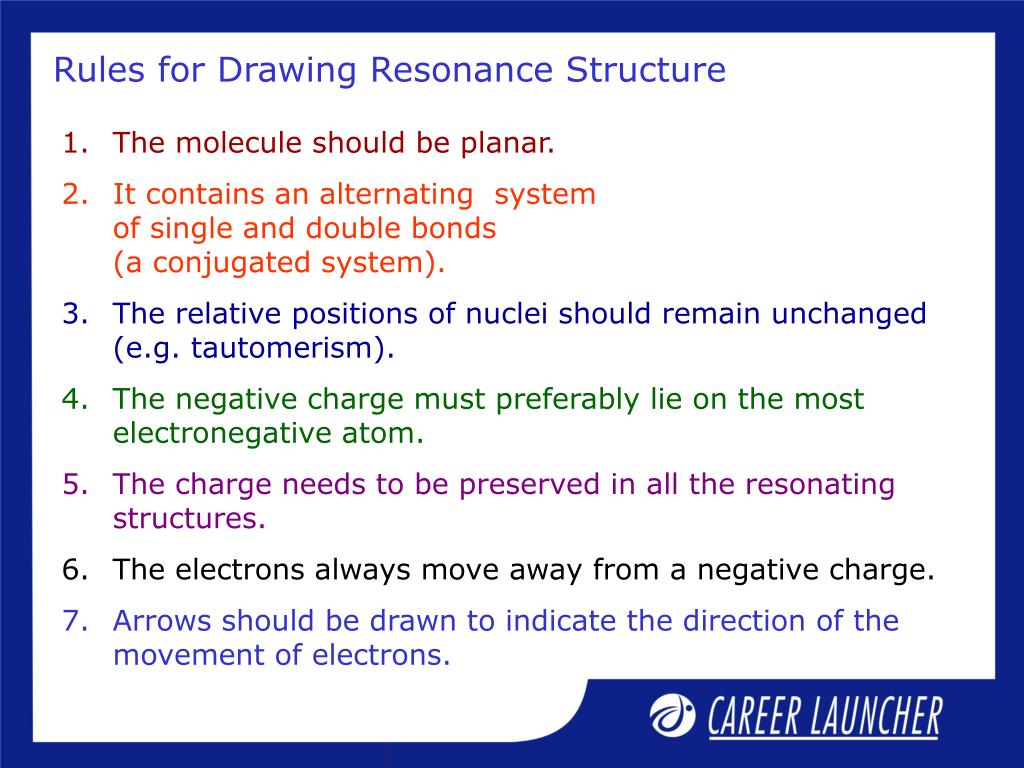
PPT Chemistry PowerPoint Presentation, free download ID378725
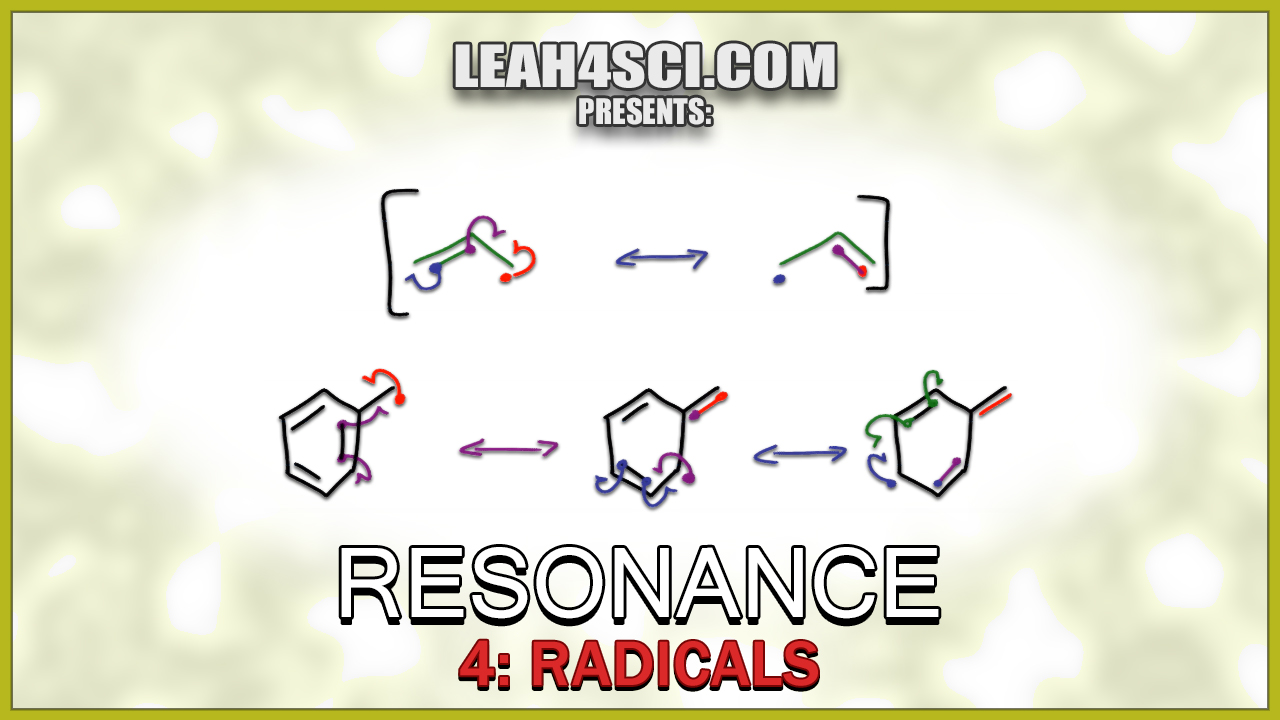
Drawing Radical Resonance for Allylic and Benzylic Radicals Tutorial Video
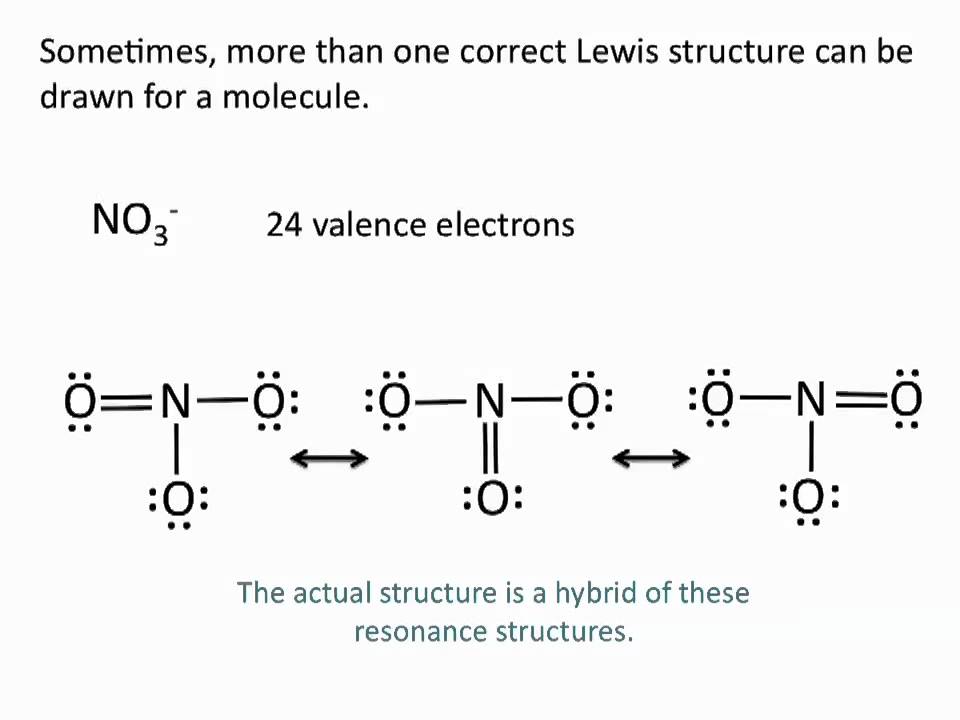
Drawing Lewis Structures Resonance Structures Chemistry Tutorial
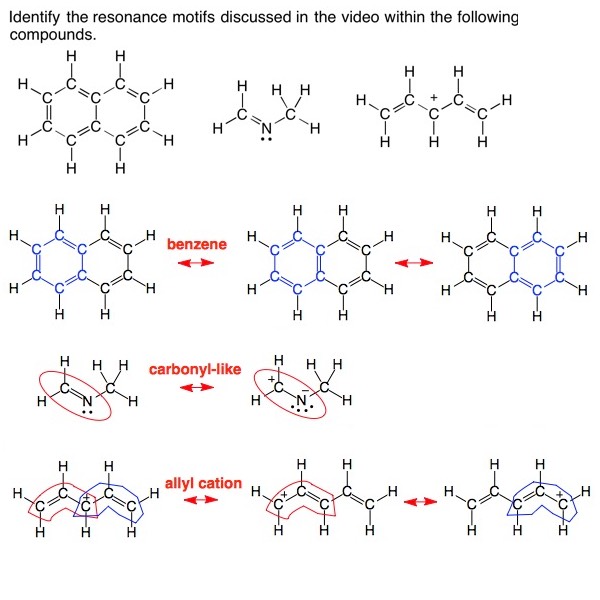
Drawing Resonance Structures (28 min) Organic Chemistry Help eMmediately
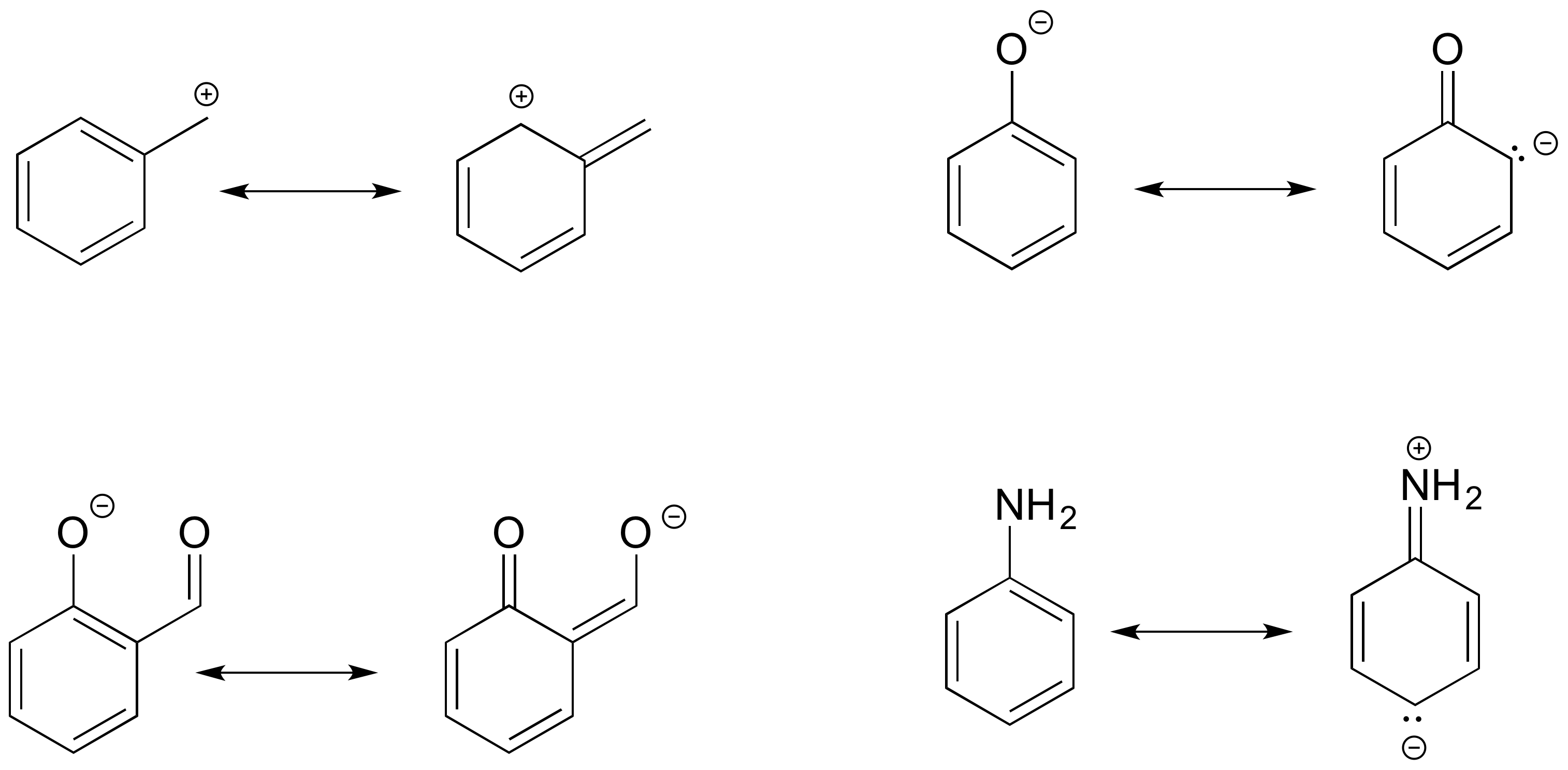
2.3 Resonance Chemistry LibreTexts

How to Draw Resonance Contributors MCC Organic Chemistry

Resonance Structures, Basic Introduction How To Draw The Resonance
Draw The Lewis Structure & Resonance.
Resonance Exists Only When A Lewis Structure Has Multiple Bonds And An Adjacent Atom With At Least One Lone Pair.
Use The Concept Of Resonance To Explain Structural Features Of Molecules And Ions.
( Keep In Mind That All The Rules Applied To Lewis Structures Still Apply Here!) All Resonance Structures Must Have The Same Atom Connectivity And Only Differ In The Electron Arrangement.
Related Post: