Drawing Resonance Forms
Drawing Resonance Forms - Learn for free about math, art, computer programming, economics, physics, chemistry, biology, medicine, finance, history, and more. Use the concept of resonance to explain structural features of molecules and ions. Want to join the conversation? The real structure is a composite, or resonance hybrid, of the different forms. Only bonds to the same atoms change from form to form. This real structure (the resonance hybrid) takes its character from the average of all the individual resonance contributors. Web when learning to draw and interpret resonance structures, there are a few basic guidelines to help. After completing this section, you should be able to. We can draw two or more lewis structures for some molecules and polyatomic ions, without changing the position of atoms in the structure. Web guidelines for drawing resonance forms. The real structure is a composite, or resonance hybrid, of the different forms. After completing this section, you should be able to. These structures are called resonance structures or contributing structures. Web 3 min read. It explains how to identify the major resonance contributor as well as the minor. This real structure (the resonance hybrid) takes its character from the average of all the individual resonance contributors. 341k views 11 years ago. Not all resonance forms are of equal importance. Individual resonance forms are imaginary, not real. Web when learning to draw and interpret resonance structures, there are a few basic guidelines to help. They are used when there is more than one way to place double bonds and lone pairs on atoms. Introduction to resonance structures, when they are used, and how they are drawn. Where the octet rule is observed, all forms obey it. Web a resonance form is another way of drawing a lewis dot structure for a given compound. Web. Web a resonance form is another way of drawing a lewis dot structure for a given compound. Web this organic chemistry video tutorial provides a basic introduction into drawing resonance structures. 1) there is only one real structure for each molecule or ion. So how do we evaluate how “important” each resonance structure is? This is like holding your hat. This is like holding your hat in either your right hand or your left. Determine the relative stability of resonance structures using a set of rules. 2.5 rules for resonance forms. These structures are called resonance structures or contributing structures. Web this organic chemistry video tutorial provides a basic introduction into drawing resonance structures. After completing this section, you should be able to. The real structure is a composite, or resonance hybrid, of the different forms. Not all resonance forms are of equal importance. Web guidelines for drawing resonance forms. This is like holding your hat in either your right hand or your left. It explains how to identify the major resonance contributor as well as the minor. Resonance is a mental exercise and method within the valence bond theory of bonding that describes the delocalization of electrons within molecules. We can draw two or more lewis structures for some molecules and polyatomic ions, without changing the position of atoms in the structure. Web. 2) do not break single bonds. Web guidelines for drawing resonance forms. The following rules should be helpful: Web draw the indicated number of resonance forms for each of the following substances: Web a resonance form is another way of drawing a lewis dot structure for a given compound. This is like holding your hat in either your right hand or your left. After completing this section, you should be able to. Determine the relative stability of resonance structures using a set of rules. In this case, only the electron distribution differs from one structure to another. The real structure is a composite, or resonance hybrid, of the different. This real structure (the resonance hybrid) takes its character from the average of all the individual resonance contributors. The four key factors in evaluating resonance structures. Web this organic chemistry video tutorial provides a basic introduction into drawing resonance structures. Individual resonance forms are imaginary, not real. 2) do not break single bonds. Resonance structures do not change the relative positions of the atoms like your arms in the metaphor. It explains how to identify the major resonance contributor as well as the minor. 1) there is only one real structure for each molecule or ion. Not all resonance forms are of equal importance. Web 3 min read. 2.5 rules for resonance forms. Only bonds to the same atoms change from form to form. Keeping these in mind, go ahead and work on the following practice problems on drawing curved arrows, missing resonance forms, and determining the more stable resonance structure. Resonance is a mental exercise and method within the valence bond theory of bonding that describes the delocalization of electrons within molecules. The following rules should be helpful: Individual resonance forms are imaginary, not real. Web when first dealing with resonance forms, it’s useful to have a set of guidelines that describe how to draw and interpret them. The real structure is a composite, or resonance hybrid, of the different forms. They are used when there is more than one way to place double bonds and lone pairs on atoms. Individual resonance forms are imaginary, not real. Draw the resonance structures of molecules or ions that exhibit delocalization.Draw The Lewis Structures For Resonance Forms Of Acetamide Printable

How to Draw Resonance Contributors MCC Organic Chemistry

Organic Chemistry Drawing resonance forms YouTube
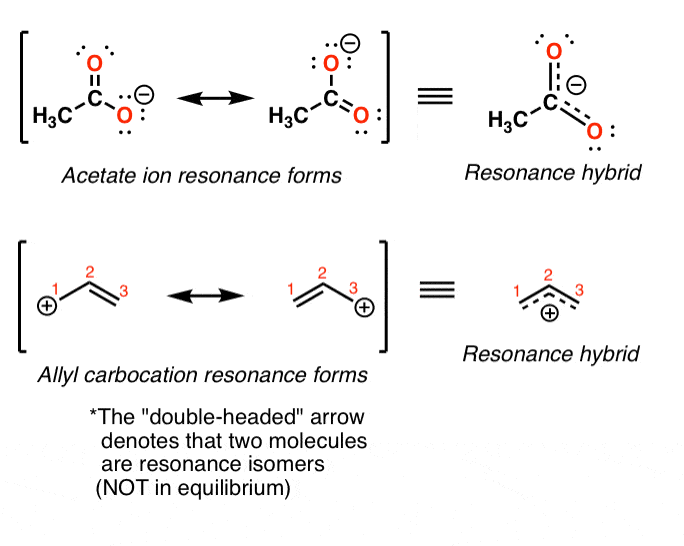
Resonance Structures 4 Rules On How To Evaluate Them, With Practice
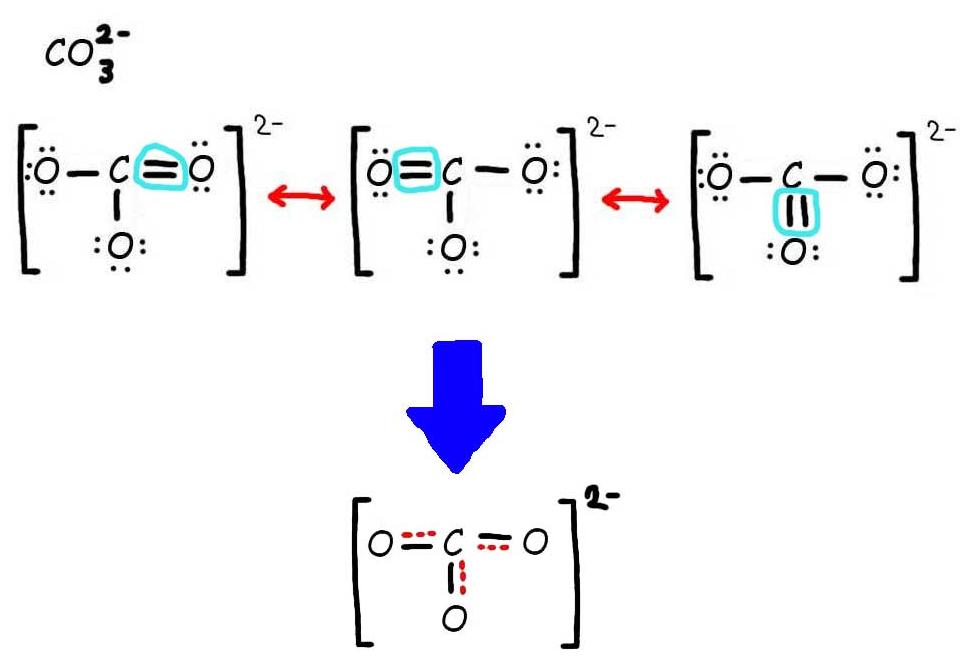
Resonance Chemwiki
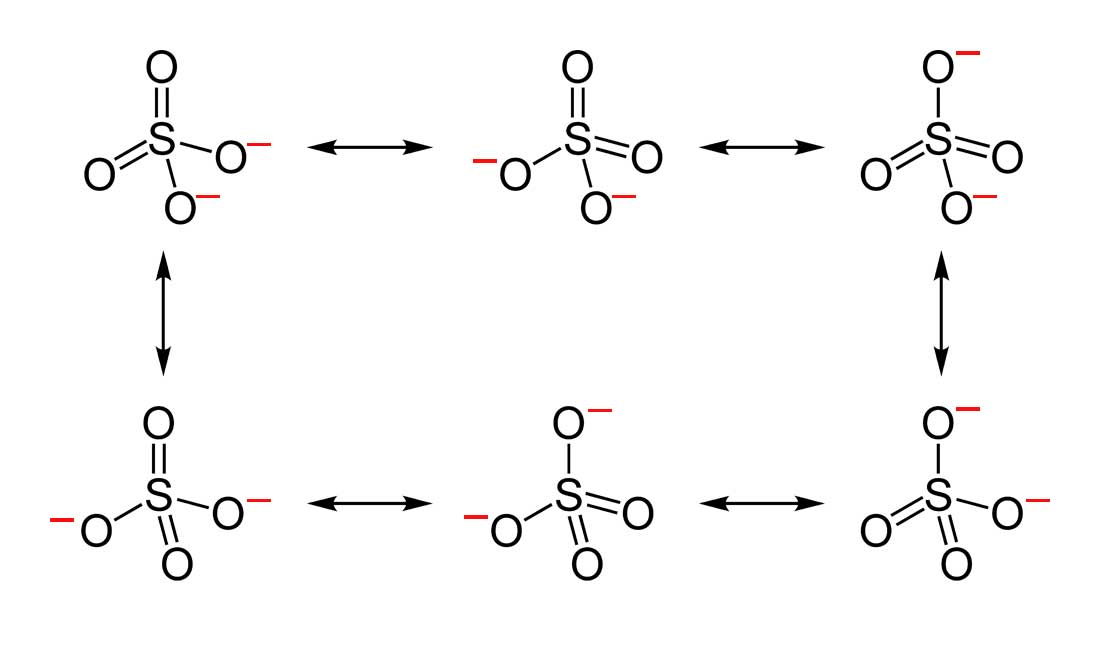
2.6 Drawing Resonance Forms Chemistry LibreTexts

drawing & ranking resonance forms YouTube
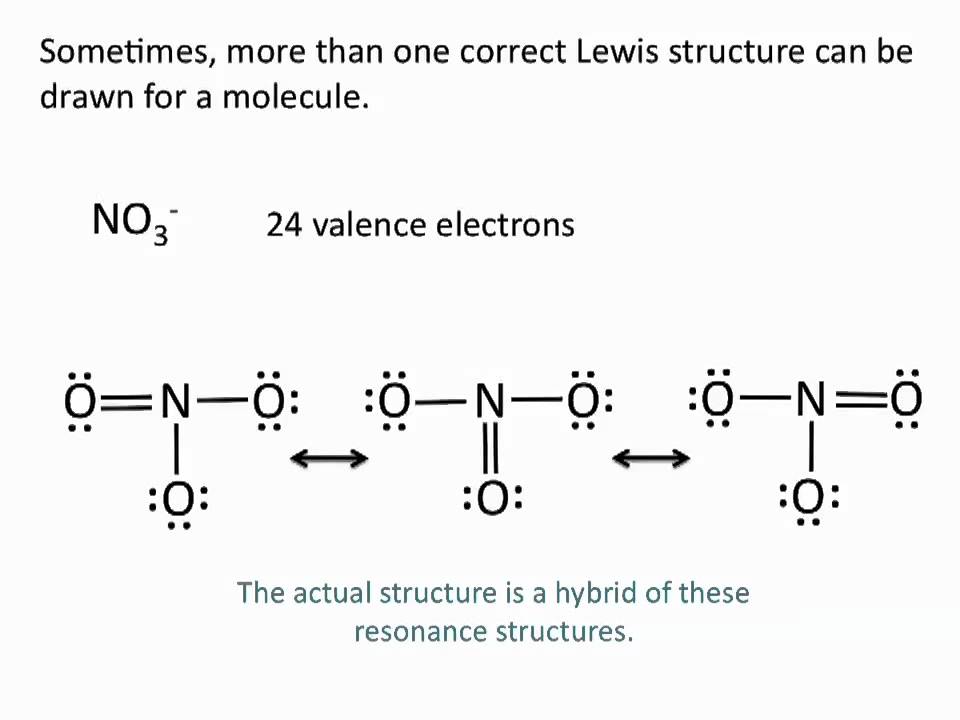
Drawing Lewis Structures Resonance Structures Chemistry Tutorial
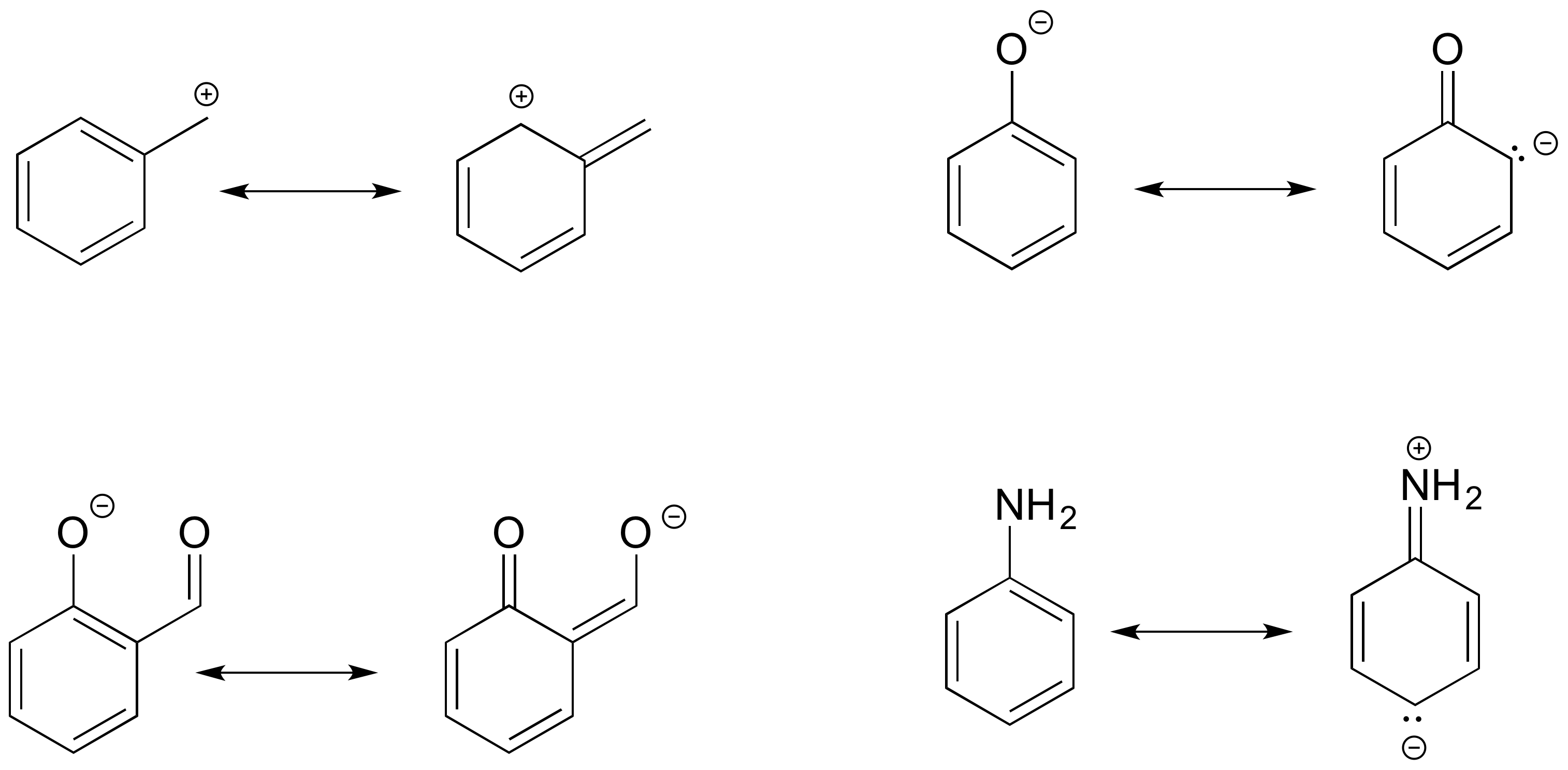
1.8 Drawing Resonance Forms Chemistry LibreTexts
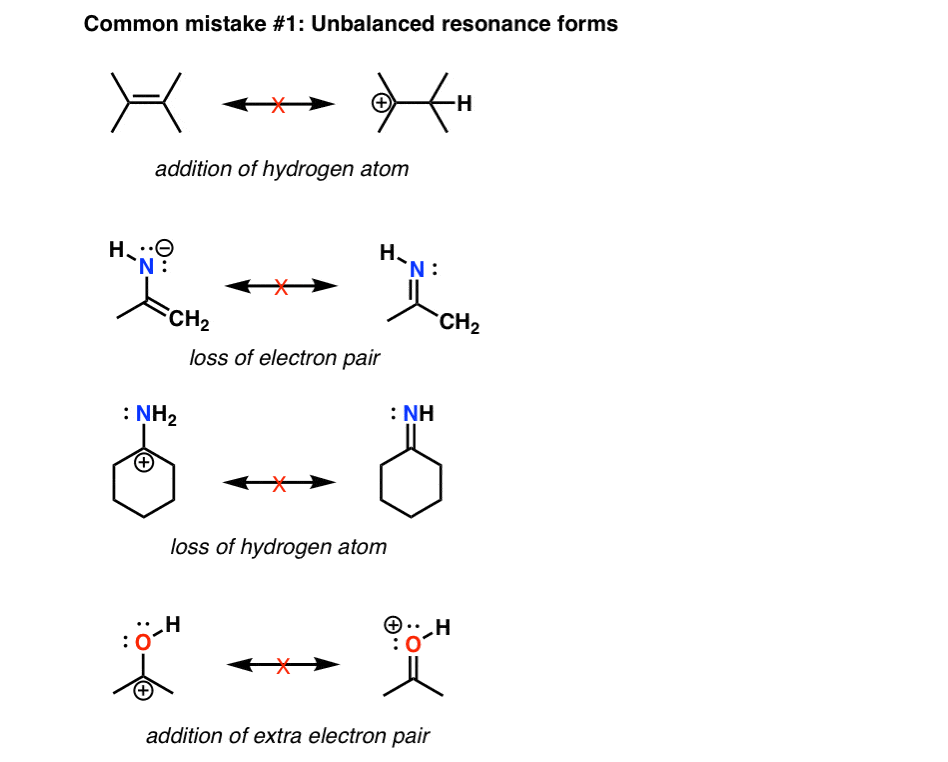
Drawing Resonance Structures 3 Common Mistakes To Avoid
Learn For Free About Math, Art, Computer Programming, Economics, Physics, Chemistry, Biology, Medicine, Finance, History, And More.
2) Do Not Break Single Bonds.
These Structures Are Called Resonance Structures Or Contributing Structures.
So How Do We Evaluate How “Important” Each Resonance Structure Is?
Related Post: