Drawing Of Fluorine
Drawing Of Fluorine - Find the number of protons, electrons, and neutrons in. To write the orbital diagram for the fluorine atom (f) first we need to write the electron configuration for just f. Fluorine is the ninth element of the periodic table. View drawing of fluorine videos. Orbital is the region of space around the nucleus of an atom where electrons are found. Start by sketching the nucleus, and then draw the two electron shells. Web fluorine is the most electronegative element because it has 5 electrons in its 2p shell. Fluorine is extremely reactive, as it reacts with all other elements except for. 26k views 4 years ago. Fluorine's atomic electron configuration is 1s 2 2s 2 2p 5 (see figure 2). Fluorine's atomic electron configuration is 1s 2 2s 2 2p 5 (see figure 2). We’ll use a bohr diagram to. Find the number of protons, electrons, and neutrons in. The electron configuration of fluorine is [ he] 2s 2 2p 5, if the electron arrangement is through orbitals. It is the lightest halogen and exists at standard conditions as a. Orbital is the region of space around the nucleus of an atom where electrons are found. Thus, its atomic mass is 19. Web steps of drawing lewis structure of f 2 molecule. Web fluorine is the most electronegative element on the periodic table, which means that it is a very strong oxidizing agent and accepts other elements' electrons. Carlos clarivan. The atomic number of each element increases by one, reading from left to right. Now it is possible for some of the elements of the second period to not have eight electrons. Number of steps can be changed according the complexity of the molecule or ion. Web to draw lewis structures for molecules and polyatomic ions with one central atom.. 13k views 2 years ago. Note the electron configuration for reference and follow the three essential rules: Web fluorine is a chemical element; Web for example, consider fluorine and sulfur. Here, we will draw the bohr diagram of the fluorine atom with some simple steps. This diagram shows how the electrons in the fluorine atom are arranged in different orbitals. Web cambodia’s relocation of people from unesco site raises concerns. The atomic number of each element increases by one, reading from left to right. Members of a group typically have similar properties and electron configurations in their outer shell. 9.7k views 2 years ago. Web fluorine is a highly reactive element that forms a diatomic molecule with the formula f2. Web fluorine is the most electronegative element on the periodic table, which means that it is a very strong oxidizing agent and accepts other elements' electrons. Thus, its atomic mass is 19. Web the fluorine orbital diagram is a graphical representation of the electron. As shown, fluorine has nine protons (i.e., 9p) and ten neutrons (i.e., 10n). The valence electrons are the electrons in the outermost shell. When we draw a lewis structure, guidelines are given. Here, we will draw the bohr diagram of the fluorine atom with some simple steps. Note the electron configuration for reference and follow the three essential rules: View drawing of fluorine videos. Web fluorine is a chemical element; Thus, its atomic mass is 19. 9), the most common isotope of the element fluorine. The valence electrons are the electrons in the outermost shell. Web the arrangement of electrons in fluorine in specific rules in different orbits and orbitals is called the electron configuration of fluorine. It is the lightest halogen and exists at standard conditions as a highly toxic, pale yellow diatomic gas. We’ll use a bohr diagram to. Web cambodia’s relocation of people from unesco site raises concerns. Web to draw the. Steps to draw the bohr model of fluorine atom. Web fluorine is a highly reactive element that forms a diatomic molecule with the formula f2. Web the arrangement of electrons in fluorine in specific rules in different orbits and orbitals is called the electron configuration of fluorine. The nucleus consists of 9 protons (red) and 10 neutrons (orange). Members of. Start by sketching the nucleus, and then draw the two electron shells. Web so for elements like carbon, nitrogen, oxygen, fluorine, understanding the octet rule is going to help you when you're drawing dot structures. Web to draw the fluorine bohr model, note the 9 protons, 10 neutrons, and 9 electrons. When we draw a lewis structure, guidelines are given. In this video we'll look at the atomic structure and bohr model for the fluorine atom (f). Now it is possible for some of the elements of the second period to not have eight electrons. Steps to draw the bohr model of fluorine atom. Web steps of drawing lewis structure of f 2 molecule. A horizontal row in the periodic table. It has symbol f and atomic number 9. Number of steps can be changed according the complexity of the molecule or ion. Fluorine has 2 electrons in its. Note the electron configuration for reference and follow the three essential rules: 9.7k views 2 years ago. Drawing of fluorine stock illustrations. Chhem hay, 37, stands at a main door of her house under construction at run ta ek village in siem reap province, cambodia, on april 2, 2024.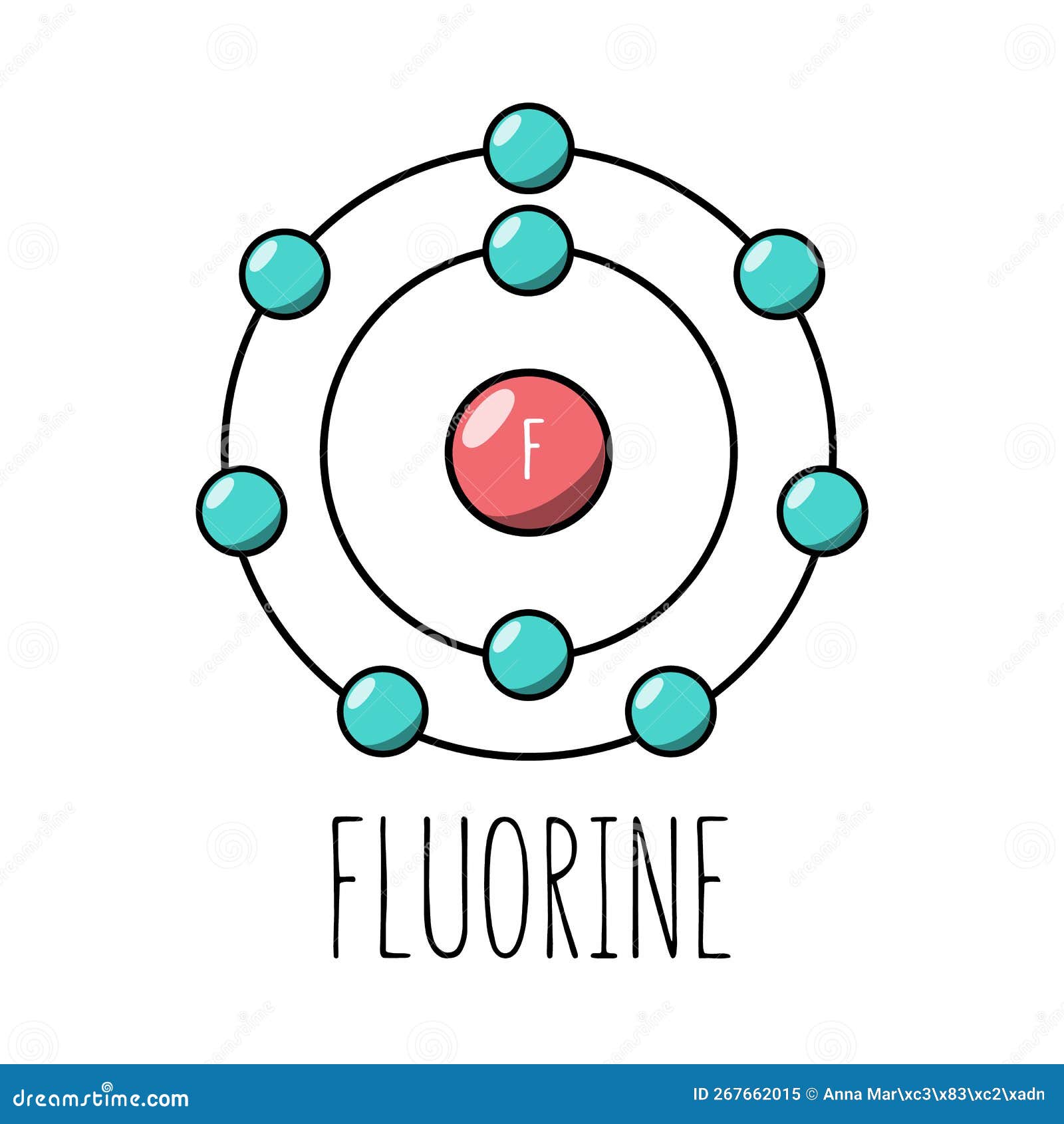
Fluorine atom Bohr model stock vector. Illustration of flat 267662015

Fluorine Atom Diagram
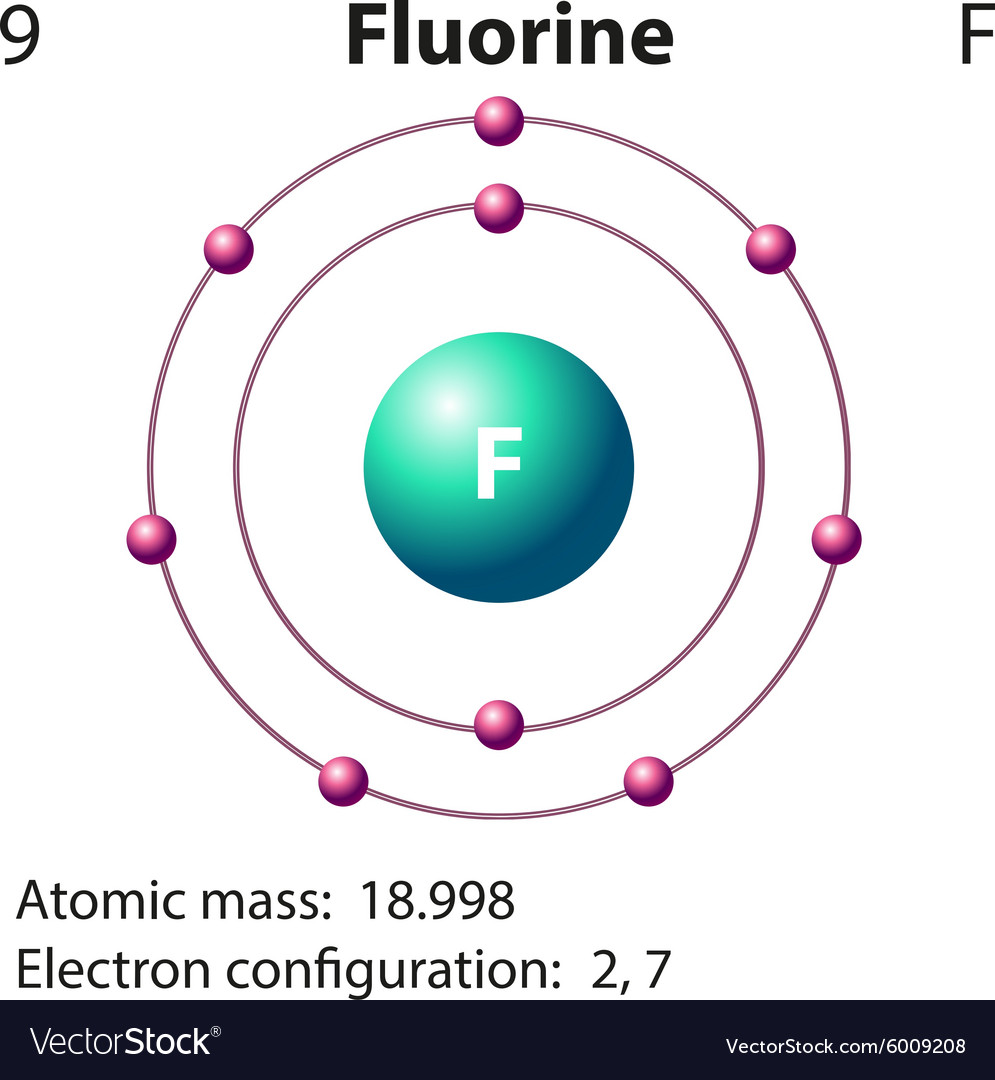
Diagram representation of the element fluorine Vector Image
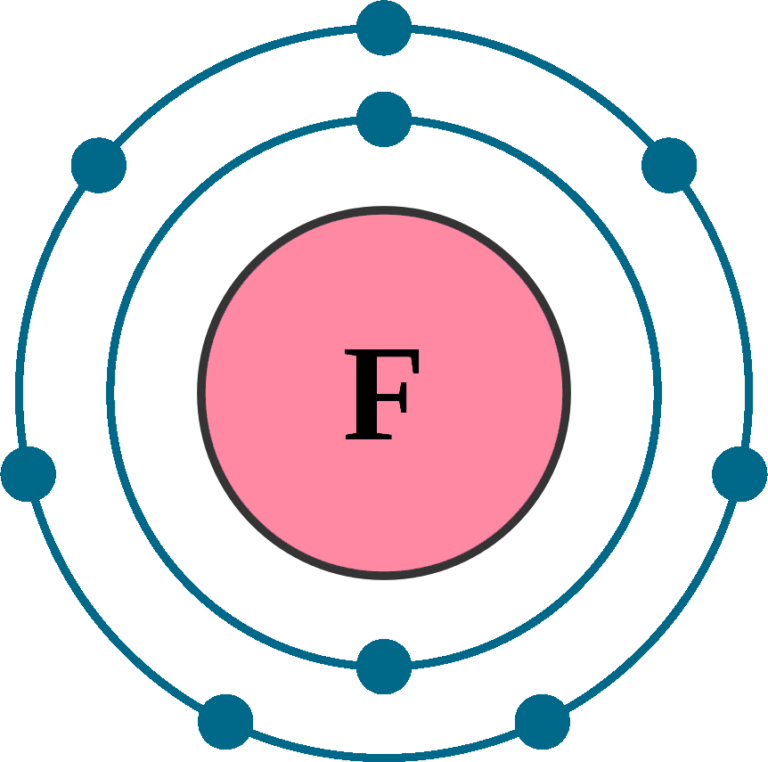
Fluorine F (Element 9) of Periodic Table Elements Flash Cards
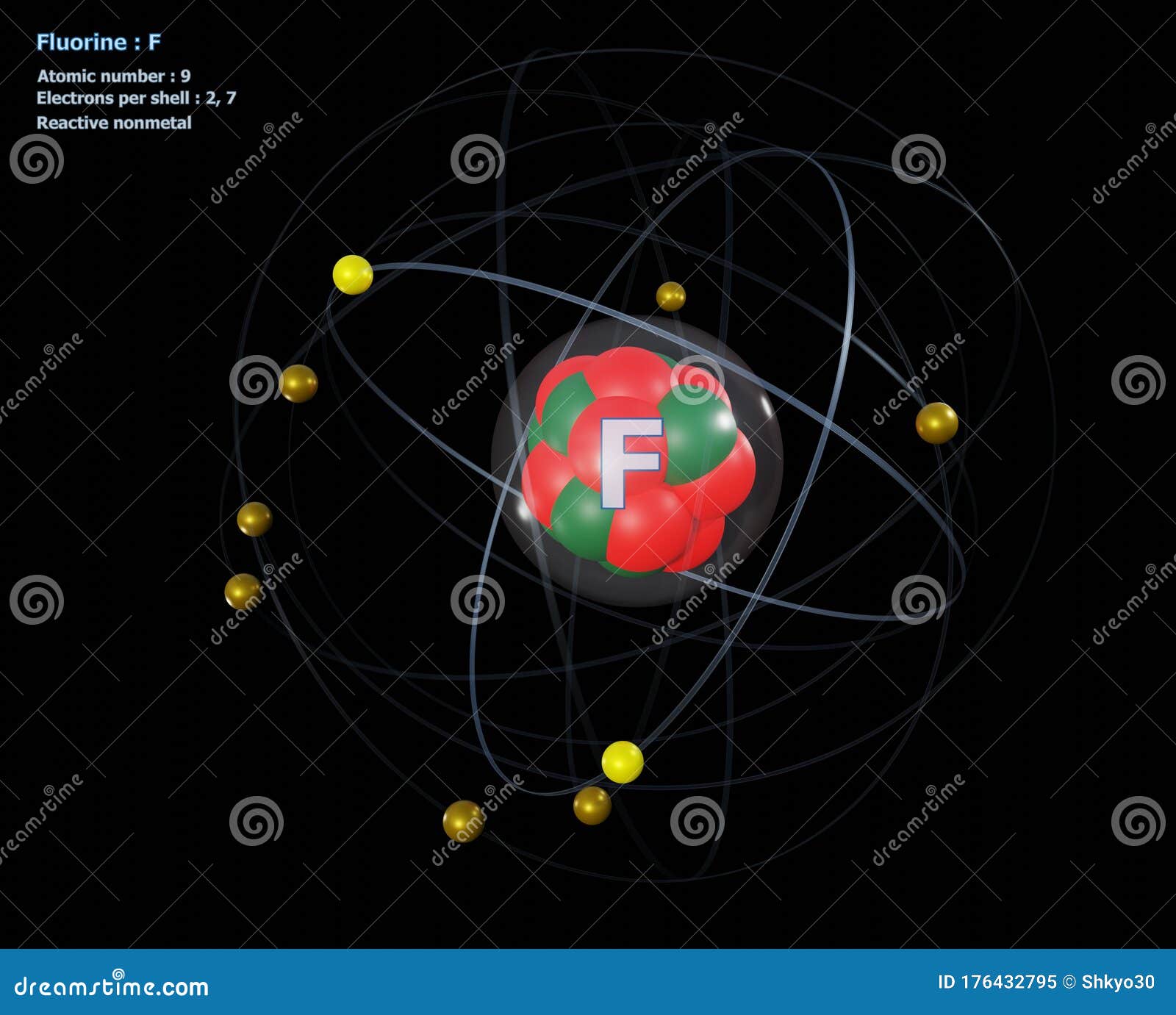
Atom of Fluorine with Detailed Core and Its 9 Electrons Stock
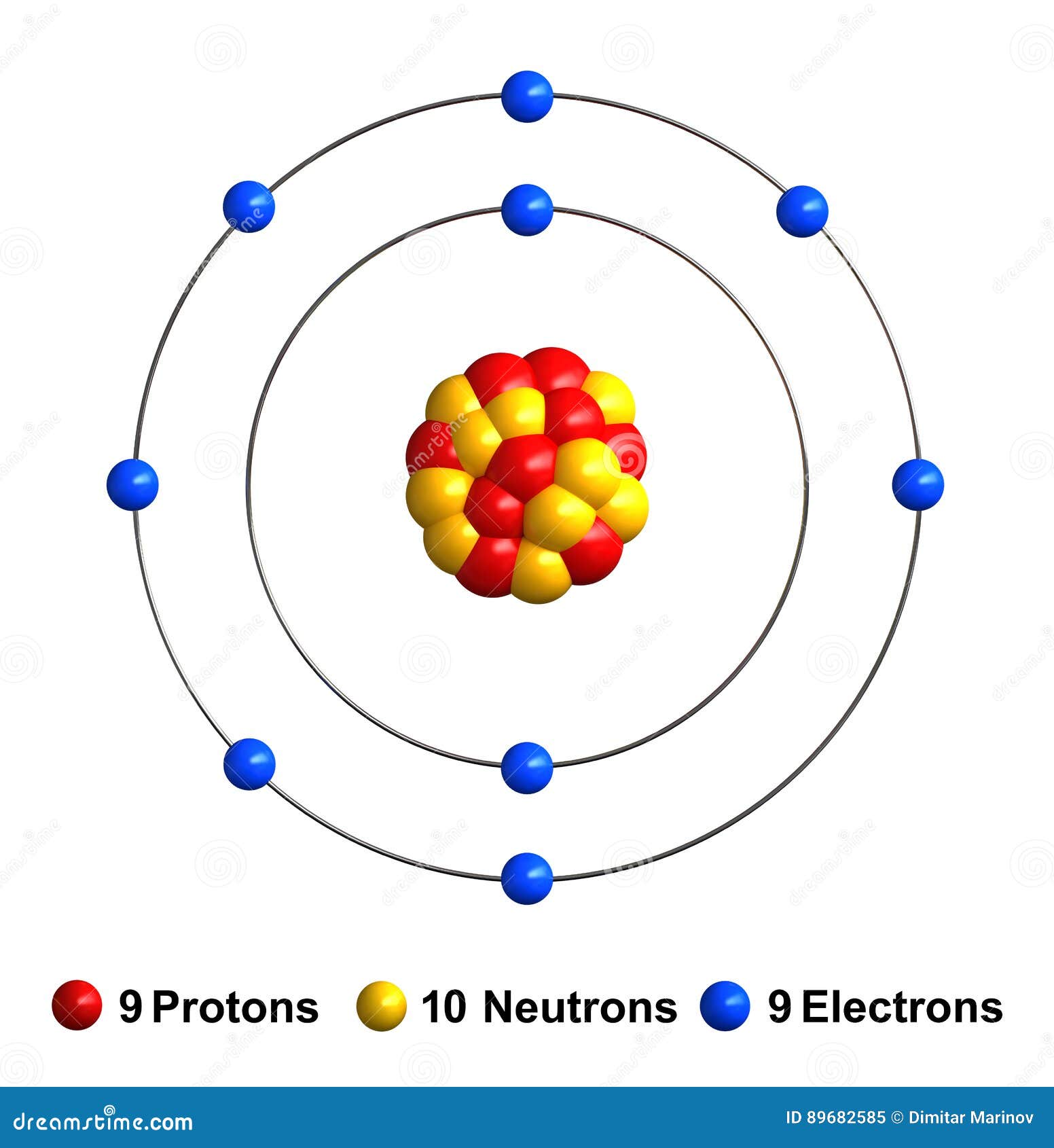
Fluorine stock illustration. Illustration of protons 89682585
![[DIAGRAM] Electron Dot Diagram Of Fluorine](http://www.benjamin-mills.com/science/GCSE/9-F-fluorine-electron.png)
[DIAGRAM] Electron Dot Diagram Of Fluorine

Fluorine, atomic structure Stock Image C013/1510 Science Photo

fluorine orbital diagram Herbalned

Fluorine molecule, illustration Stock Photo Alamy
A Vertical Column In The Periodic Table.
9), The Most Common Isotope Of The Element Fluorine.
Members Of A Group Typically Have Similar Properties And Electron Configurations In Their Outer Shell.
Web For Example, Consider Fluorine And Sulfur.
Related Post: