Drawing Molecular Orbitals
Drawing Molecular Orbitals - Web a molecular orbital diagram, or mo diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals (lcao) method in particular. Web combine the two sodium valence atomic orbitals to produce bonding and antibonding molecular orbitals. Both h a 2 a + and h a 2 a − are equally stable. Determine the total number of valence electrons in the na 2 − ion. Web how might one draw atomic and molecular orbital diagrams? Which among the following is true with respect to the given species? The applications of the mo theory extend beyond the limitations of the valence shell electron pair repulsion (vsepr) model and the valence bond theory. Valence bond theory is able to explain many aspects of bonding, but not all. Both h a 2 a + and h a 2 a − do not exist. How to draw a molecular orbital diagram. This gives you the total number of electrons you will have to distribute among the molecular orbitals you form. Web combine the two sodium valence atomic orbitals to produce bonding and antibonding molecular orbitals. We can describe the electronic structure of diatomic molecules by applying molecular orbital theory to the valence electrons of the atoms. Web drawing molecular orbital diagrams.. This chemistry video tutorial provides a basic introduction into molecular orbital theory. Both h a 2 a + and h a 2 a − do not exist. Web how might one draw atomic and molecular orbital diagrams? We’ll compare them with the molecular orbitals for (linear) hexatriene. Both h a 2 a + and h a 2 a − are. Just like the atomic orbitals, molecular orbitals (mo) are used to describe the bonding in molecules by applying the group theory. Get out your pencil (and eraser) because we are about to learn how to draw atomic orbitals. H a 2 a + and h a 2 a −. Build the house (orbitals) first, and fill it with people (electrons). Valence bond theory is able to explain many aspects of bonding, but not all. The applications of the mo theory extend beyond the limitations of the valence shell electron pair repulsion (vsepr) model and the valence bond theory. Get a 10 bullets summary of the topic. The structural formula editor is surround by three toolbars which contain the tools you. The applications of the mo theory extend beyond the limitations of the valence shell electron pair repulsion (vsepr) model and the valence bond theory. To complement this theory, we use another called the molecular orbital (mo) theory. Web learn to draw molecular orbital electron configuration energy diagrams. Molecular orbital theory is a more sophisticated model for understanding the nature of. Both h a 2 a + and h a 2 a − do not exist. Web today, let’s go through how to draw out the molecular orbitals of benzene. Web a molecular orbital diagram, or mo diagram, is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of. Valence bond theory is able to explain many aspects of bonding, but not all. Determine the atomic orbitals of your atoms. Which among the following is true with respect to the given species? Get a 10 bullets summary of the topic. It describes the formation of. Web the molecular orbital (mo) theory is a powerful and extensive approach which describes electrons as delocalized moieties over adjacent atoms. Web combine the two sodium valence atomic orbitals to produce bonding and antibonding molecular orbitals. The site includes opportunities to practice filling in electrons, attaching the names/symbols of mos, and matching orbital overlap drawings to mos. The 7 rules. We’ll compare them with the molecular orbitals for (linear) hexatriene. The applications of the mo theory extend beyond the limitations of the valence shell electron pair repulsion (vsepr) model and the valence bond theory. Web the molecular orbital (mo) theory is a powerful and extensive approach which describes electrons as delocalized moieties over adjacent atoms. This chemistry video tutorial provides. Give them a try here: Web the molecular orbital (mo) theory is a powerful and extensive approach which describes electrons as delocalized moieties over adjacent atoms. Build the house (orbitals) first, and fill it with people (electrons) second. Both h a 2 a + and h a 2 a − are equally stable. Web today, let’s go through how to. Web today, let’s go through how to draw out the molecular orbitals of benzene. To complement this theory, we use another called the molecular orbital (mo) theory. Determine the atomic orbitals of your atoms. So the atomic orbital diagram is simply those orbitals in that order of energy. The 7 rules of drawing molecular orbitals. Both h a 2 a + and h a 2 a − are equally stable. Determine the total number of valence electrons in the na 2 − ion. We’ll compare them with the molecular orbitals for (linear) hexatriene. Here are the 7 rules you need to know about how to draw molecular orbitals. Get out your pencil (and eraser) because we are about to learn how to draw atomic orbitals. Valence bond theory is able to explain many aspects of bonding, but not all. This gives you the total number of electrons you will have to distribute among the molecular orbitals you form. It describes the formation of. Get a 10 bullets summary of the topic. This chemistry video tutorial provides a basic introduction into molecular orbital theory. Web combine the two sodium valence atomic orbitals to produce bonding and antibonding molecular orbitals.
The Pi Molecular Orbitals of Butadiene And How To Draw Them

Radial and Angular Parts of Atomic Orbitals Chemistry LibreTexts

Molecular Orbital Diagrams simplified by Megan Lim Medium
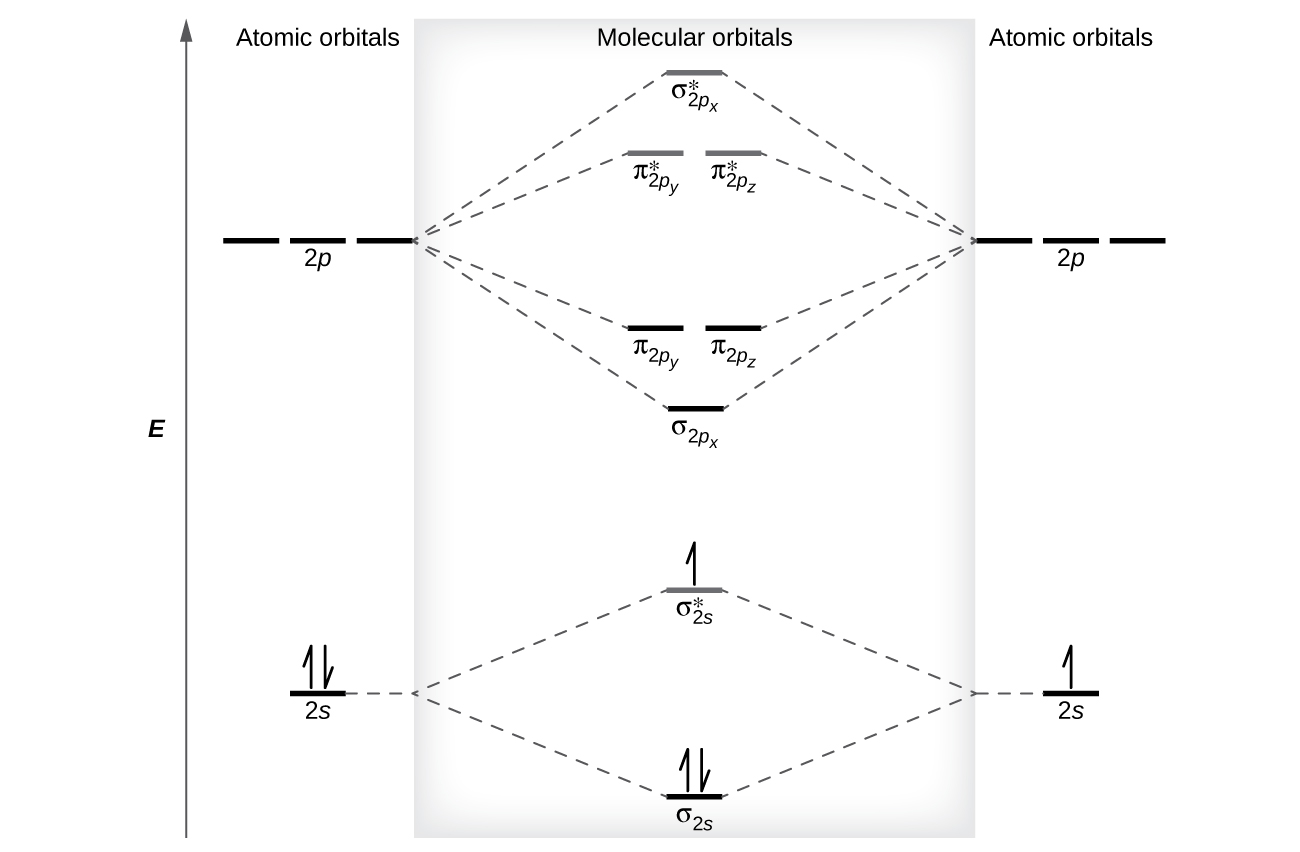
Molecular Orbital Theory · Chemistry
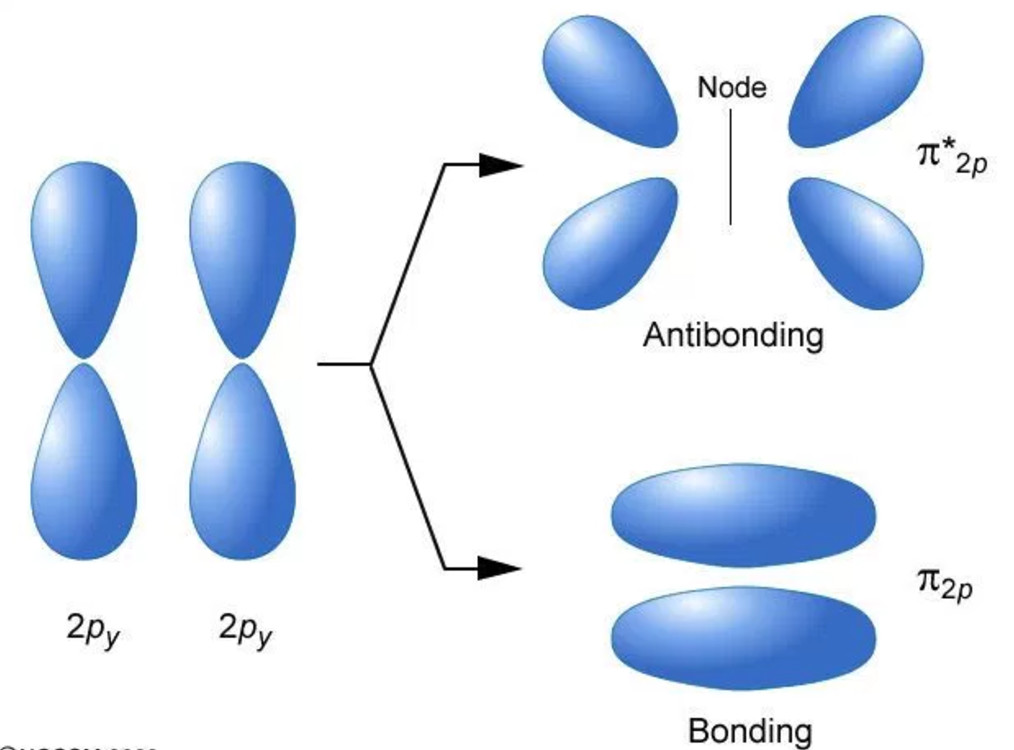
Types of Molecular Orbital Formed Chemical Bonding and Molecular
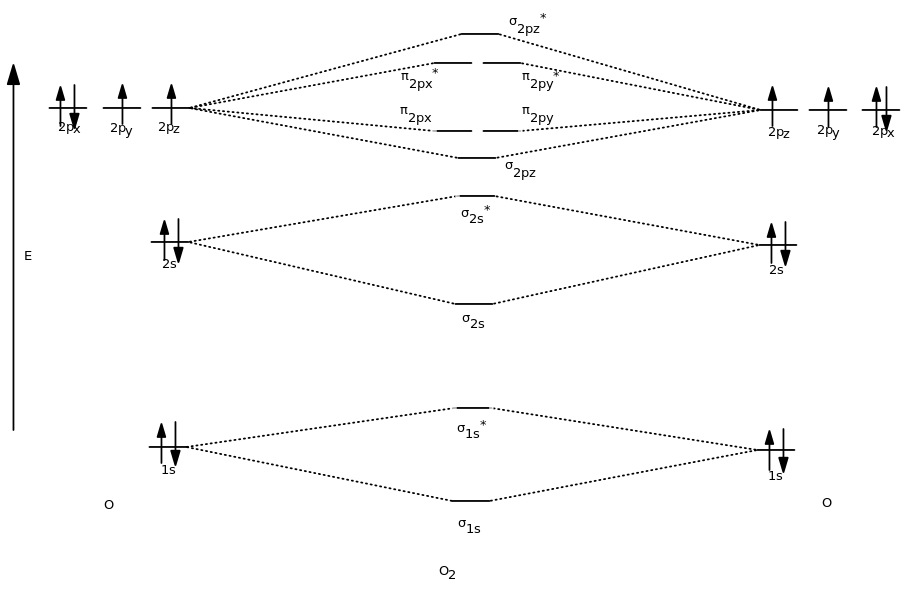
Drawing Atomic and Molecular Orbitals Diagrams for Molecules Organic
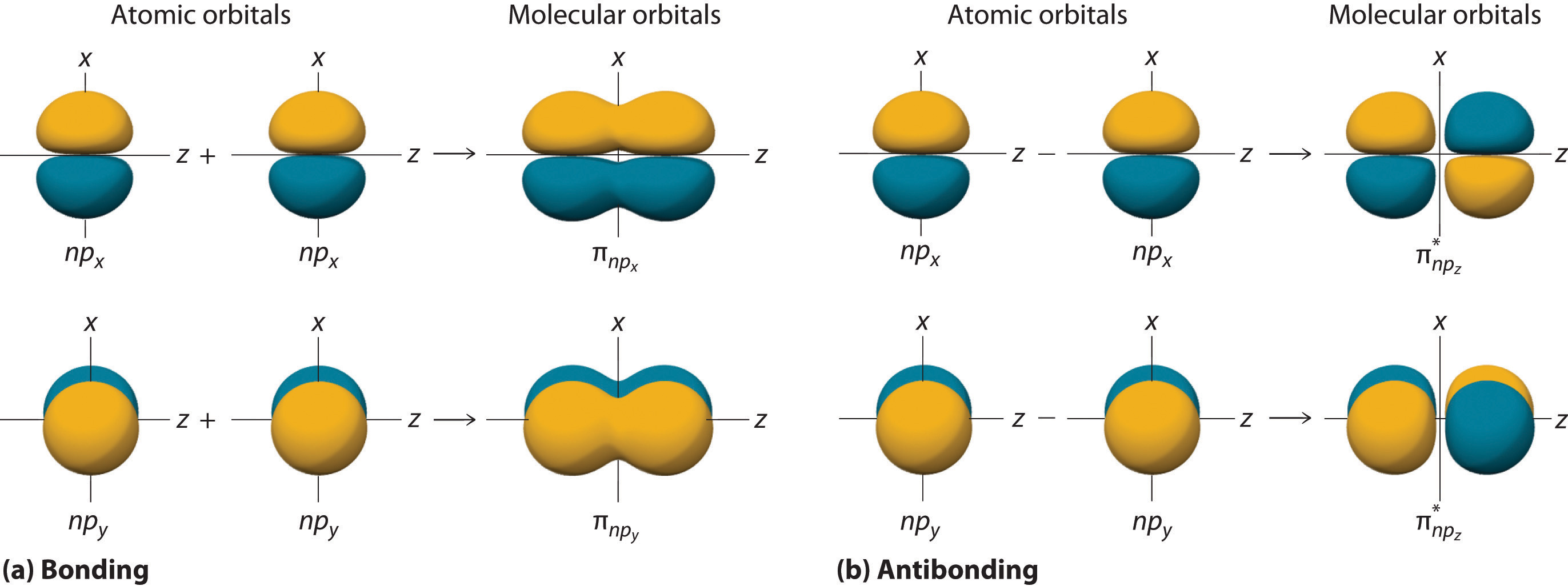
3.1.7 Molecular Orbitals Chemistry LibreTexts
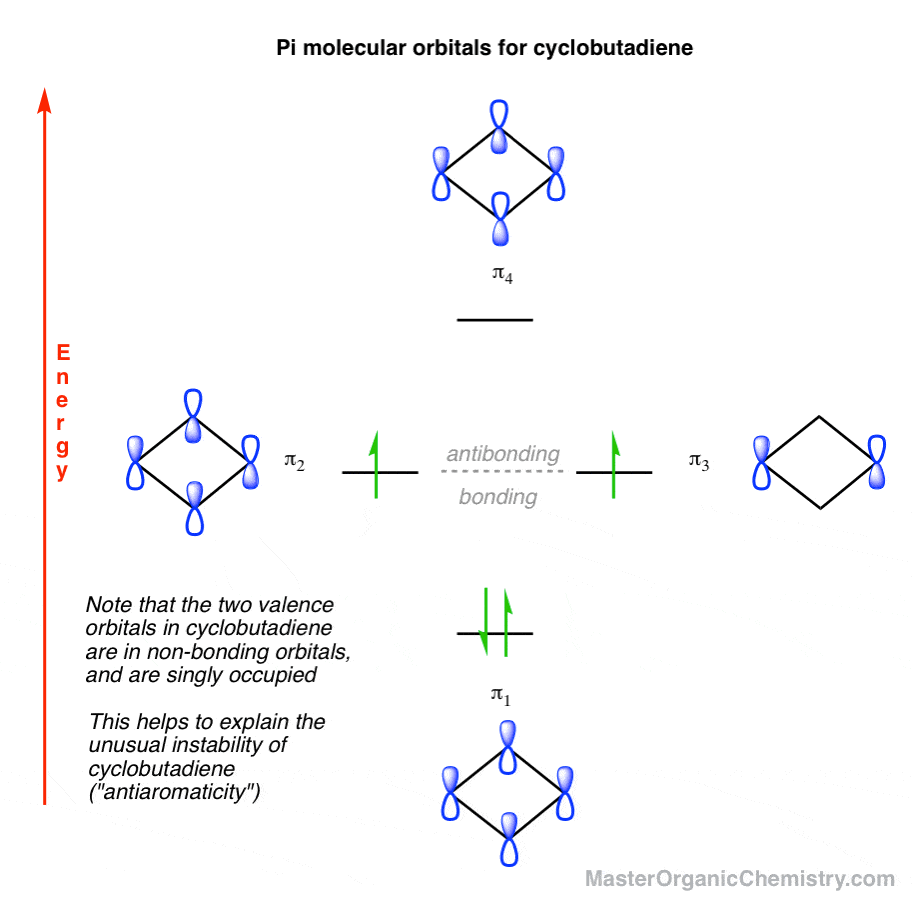
Cyclobutadiene How to Build Up The Molecular Orbital Diagram

Molecular orbital diagram for nitrogen monoxide, the nitrosyl cation

8.3 Development of Quantum Theory CHEM 1114 Introduction to Chemistry
We Can Describe The Electronic Structure Of Diatomic Molecules By Applying Molecular Orbital Theory To The Valence Electrons Of The Atoms.
Web Drawing Molecular Orbital Diagrams.
Get A 10 Bullets Summary Of The Topic.
Web How Might One Draw Atomic And Molecular Orbital Diagrams?
Related Post: