Draw Valine At Physiological Ph
Draw Valine At Physiological Ph - Draw the predominant form of a given amino acid in a solution of known ph, given the isoelectric point of the amino acid. Draw the predominant form of valine when the ph = 7.4 b. Web the ph at which the net positive and negative charges in a sample of an amino acid are evenly balanced is the amino acid/s isoelectric point or pi. Low ph pi high ph d. Note the diprotic amino acid, alanine. Describe, briefly, how a mixture of amino acids may be separated by paper electrophoresis. Web in the absence of pathological states, the ph of the human body ranges between 7.35 to 7.45, with the average at 7.40. We're talking about veiling this amino acid with on our group with two branch methyl groups like this. Draw valine at physiological ph. Web structures of amino acids at ph 7.0 author: Draw valine at physiological ph. Web for example, at certain ph's, some amino acids will be zwitterionic. Example 26.1 draw the structure for the anion formed when glycine (at neutral ph) reacts with a base. Draw the structure for the anion formed when valine (at neutral ph) reacts with a base. Draw the structure for the anion formed when valine. Draw the structure for the anion formed when valine (at neutral ph) reacts with a base. The particular ph at which a given amino acid exists in solution as a zwitterion is called the isoelectric point (pi). Describe, briefly, how a mixture of amino acids may be separated by paper electrophoresis. Therefore the \(\ce{h^+}\) will add to the carboxylate ion. Web draw the structure for the cation formed when valine (at neutral ph) reacts with an acid. Draw the predominant form of valine when the ph = 12.0 d. Example 26.1 draw the structure for the anion formed when glycine (at neutral ph) reacts with a base. Low ph pi high ph d. Web at ph = 3.52, the \(\ce{h^+}\). Web given that amino acid requirements vary with the duration of the diet regimen, genetic background and physiological state of pigs, the targeted application of valine is essential [98, 148]. The results of this study showed that electronic properties of the two forms of valine are similar at zero pressure. Describe, briefly, how a mixture of amino acids may be. We were talking about it at ph of one, which is really, really. Some groups are hydrophobic (e.g., branched chain and aromatic amino acids) and some hydrophilic (most others). You'll get a detailed solution from a subject matter expert that helps you learn core concepts. The particular ph at which a given amino acid exists in solution as a zwitterion. Low ph pi high ph d. Draw the structure for the anion formed when valine (at neutral ph) reacts with a base. Describe, briefly, how a mixture of amino acids may be separated by paper electrophoresis. Valine is essential in humans, meaning the body cannot synthesize it; It must be obtained from dietary sources which are food… Low ph pi high ph d. The particular ph at which a given amino acid exists in solution as a zwitterion is called the isoelectric point (pi). Draw the structure for the anion formed when valine (at neutral ph) reacts with a base. Draw the predominant form of valine when the ph = 7.4 b. We're talking about veiling this. The results of this study showed that electronic properties of the two forms of valine are similar at zero pressure. Draw valine at physiological ph. Web in addition to differences in size, these side groups carry different charges at physiological ph (e.g., nonpolar, uncharged but polar, negatively charged, positively charged); Draw the predominant form of valine when the ph =. The particular ph at which a given amino acid exists in solution as a zwitterion is called the isoelectric point (pi). Draw the predominant form of valine when the ph = 7.4 b. The amino acid structures as shown in lecture are the predominant forms at physiological ph (7.4). Example 26.1 draw the structure for the anion formed when glycine. Therefore the \(\ce{h^+}\) will add to the carboxylate ion and neutralize the negative charge. Draw the structure for the anion formed when valine (at neutral ph) reacts with a base. Describe, briefly, how a mixture of amino acids may be separated by paper electrophoresis. Web in the absence of pathological states, the ph of the human body ranges between 7.35. Draw the predominant form of a given amino acid in a solution of known ph, given the isoelectric point of the amino acid. Draw the predominant form of valine when the ph = 12.0 d. The particular ph at which a given amino acid exists in solution as a zwitterion is called the isoelectric point (pi). Some groups are hydrophobic (e.g., branched chain and aromatic amino acids) and some hydrophilic (most others). Web given that amino acid requirements vary with the duration of the diet regimen, genetic background and physiological state of pigs, the targeted application of valine is essential [98, 148]. Web in the absence of pathological states, the ph of the human body ranges between 7.35 to 7.45, with the average at 7.40. Draw the predominant form of valine when the ph = 7.4 b. Draw the predominant form of valine when the ph = 7.4 b. Draw the structure for the anion formed when valine (at neutral ph) reacts with a base. We were talking about it at ph of one, which is really, really. The amino acid will have a positive charge on the amine group left and will have an overall charge of \(+1\). Web introduction to general, organic and biochemistry. Alanine at its pi (overall charge = ) c. Web draw the structure for the cation formed when valine (at neutral ph) reacts with an acid. Draw valine at physiological ph. After completing this section, you should be able to.Solved Draw valine at physiological pH. Select Draw Rings

OneClass Modify the structure of the amino acid valine to complete the

Valine Chemical Structure. Vector Illustration Hand Drawn

Valine Chemical Structure. Vector Illustration Hand Drawn Stock Vector
SOLVEDDraw the structure for each of the following amino acids at
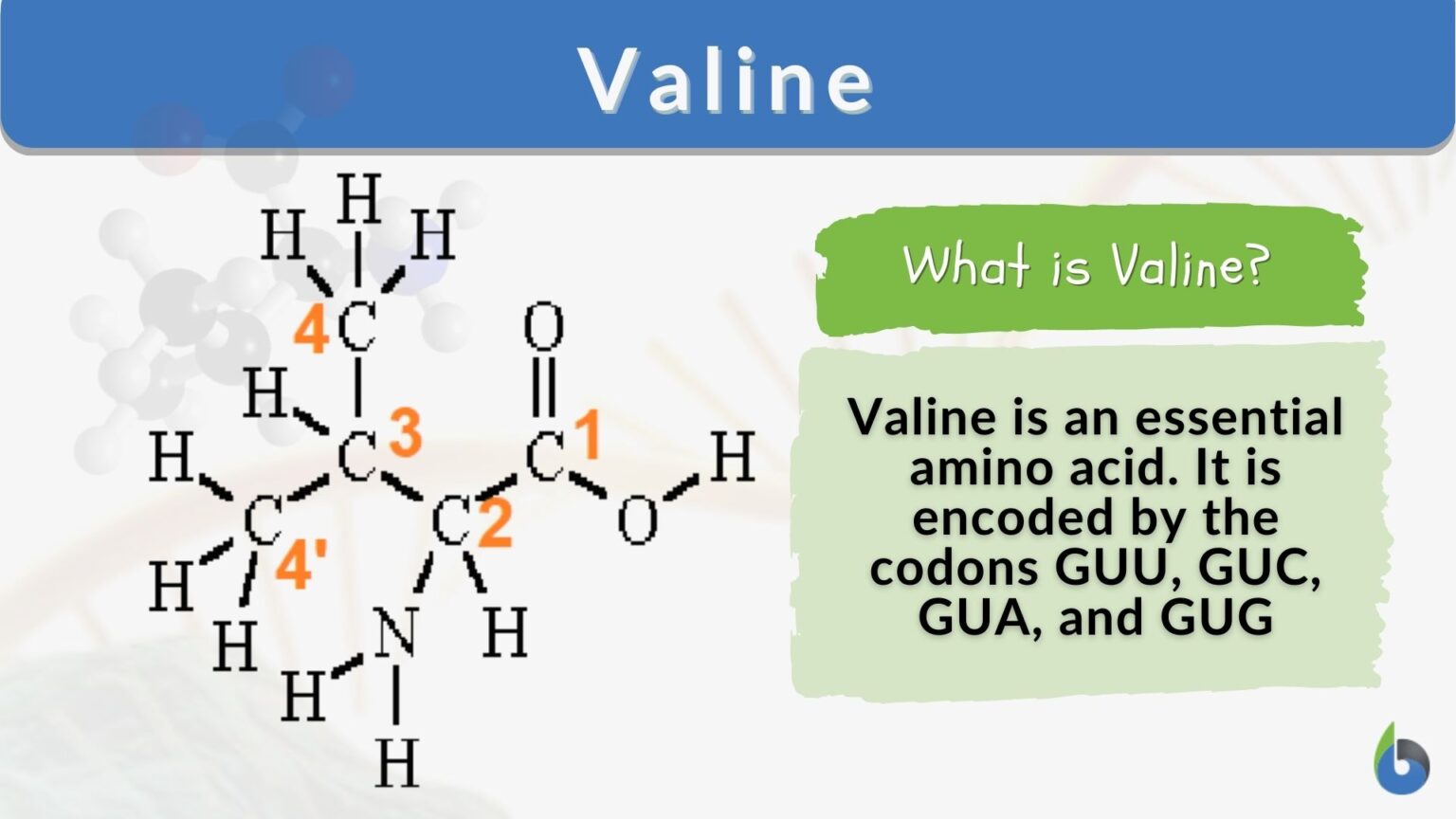
Valine Definition and Examples Biology Online Dictionary

Valine molecular structure. Valine skeletal chemical formula. Chemical
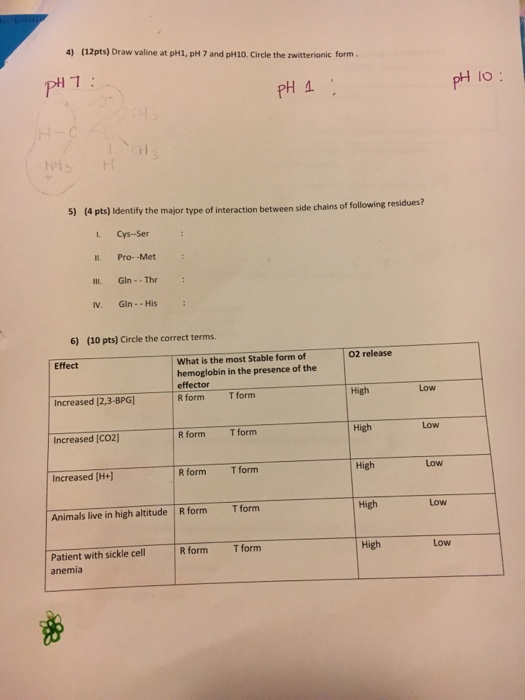
Solved Draw valine at pH1, pH 7 and pH10. Circle the
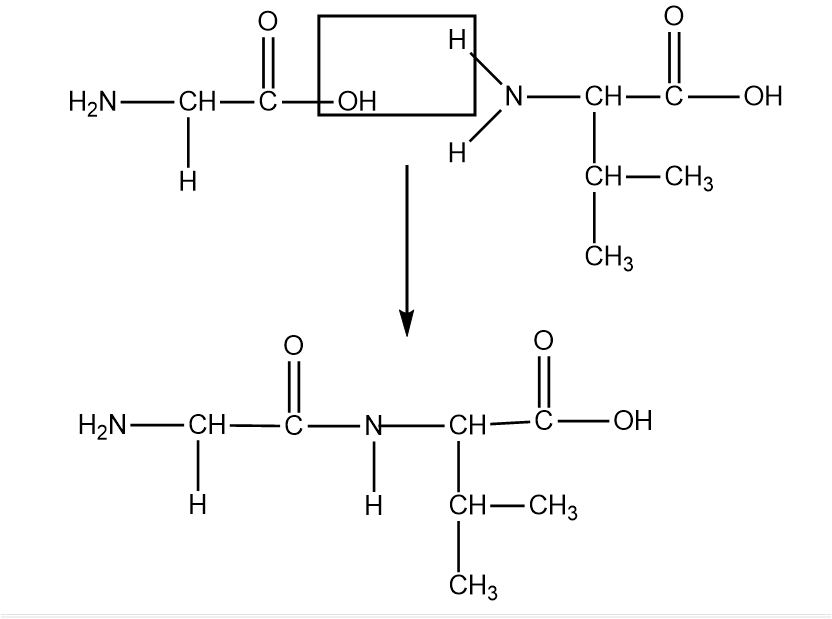
Valine Structure
Solved Draw the structure of valine at pH 1 and at pH 12.
The Results Of This Study Showed That Electronic Properties Of The Two Forms Of Valine Are Similar At Zero Pressure.
C 5 H 11 No 2.
Web The Ph At Which The Net Positive And Negative Charges In A Sample Of An Amino Acid Are Evenly Balanced Is The Amino Acid/S Isoelectric Point Or Pi.
In Animal Husbandry, Supplementation With Valine Not Only Increases Growth And Reproductive Performances, But Also Regulates Gut Microbiota And Immune.
Related Post: