Draw The Structure Of Aniline
Draw The Structure Of Aniline - Click here:point_up_2:to get an answer to your question :writing_hand:draw the resonating structure of aniline. Draw the structure of aniline. C 6 h 7 n. 23.3 structure and properties of amines. Chemspider also provides access to spectra, vendors, articles and other data sources for millions of compounds. C 6 h 7 n. This problem has been solved! The resonance structures of aniline are drawn by first displacing the lone pair of electrons on the nitrogen to the bond between c and n. Draw the structure of aniline. This results in formation of a double bond between c and n with n getting a positive charge due to the donation of electrons. Jmol._canvas2d (jsmol) jmolapplet0 [x] appletreadycallback =. The resonance structures of aniline are drawn by first displacing the lone pair of electrons on the nitrogen to the bond between c and n. A lone pair of electrons on n atom. 23.3 structure and properties of amines. It has a formula c6h5nh2 since it has 6 carbon atoms, 1 nitrogen atom and. You can search by structure or substructure, upload a structure file or draw using a molecule editor. 1 n atom joined to benzene ring. C 6 h 7 n. This problem has been solved! Web an introduction to phenylamine (aniline) | chemkey. This results in the chemical formula c 6 h 5 nh 2. Chemspider also provides access to spectra, vendors, articles and other data sources for millions of compounds. A lone pair of electrons on n atom. You can search by structure or substructure, upload a structure file or draw using a molecule editor. 1 n atom joined to benzene ring. C 6 h 7 n. Aniline is substantially less basic than methylamine, as is evident by looking at the pk a values for their respective ammonium conjugate acids (remember that the lower the pka of the conjugate acid, the weaker the base). Anilines are an industrially significant commodity chemical. The resonance structures of aniline are drawn by first displacing the. Aniline is the simplest aromatic amine. See functional group, molecular weight, properties, basicity, reaction, and uses of aniline. Consisting of a phenyl group (−c 6 h 5) attached to an amino group (−nh 2), aniline is the simplest aromatic amine. C 6 h 7 n. This results in formation of a double bond between c and n with n getting. The compound is a derivative of aniline. There are 2 steps to solve this one. This results in the chemical formula c 6 h 5 nh 2. After completing this section, you should be able to. 2 h atoms bonded to the n atom. Web find the formula and structure of aniline. See functional group, molecular weight, properties, basicity, reaction, and uses of aniline. This structure is also available as a 2d mol file or as a computed 3d sd file. The compound is a derivative of aniline. Anilines are an organic compound. Aniline, also known as aminobenzene or phenylamine, has 6 carbon (c) atoms, 7 hydrogen (h) atoms, and 1 nitrogen (n) atom in its chemical formula of c6h7n or c6h5nh2. Jmol._canvas2d (jsmol) jmolapplet0 [x] appletreadycallback =. Consisting of a phenyl group (−c 6 h 5) attached to an amino group (−nh 2), aniline is the simplest aromatic amine. See functional group,. Aniline is substantially less basic than methylamine, as is evident by looking at the pk a values for their respective ammonium conjugate acids (remember that the lower the pka of the conjugate acid, the weaker the base). Jmol._canvas2d (jsmol) jmolapplet0 [x] appletreadycallback =. Web chemspider is a free online database of chemical structures and properties. C 6 h 7 n.. Draw the structure of aniline. 23.3 structure and properties of amines. Anilines has a phenyl group attached to an amino group. It has a formula c6h5nh2 since it has 6 carbon atoms, 1 nitrogen atom and 7 hydrogen atoms. This results in formation of a double bond between c and n with n getting a positive charge due to the. Describe the geometry and bonding of simple amines. Web aniline’s molecular structure is comprised of a benzene ring (c 6 h 5) attached to an amino group (nh 2 ). This results in formation of a double bond between c and n with n getting a positive charge due to the donation of electrons. Chemspider also provides access to spectra, vendors, articles and other data sources for millions of compounds. Jmol._canvas2d (jsmol) jmolapplet0 [x] appletreadycallback =. Web find the formula and structure of aniline. Web this structure is also available as a 2d mol file or as a computed 3d sd file the 3d structure may be viewed using java or javascript. Aniline is the simplest aromatic amine. Web #k2chemistryclass #resonance #aniline #aniline_resonance #resonance_structure #chemistryformula #chemistry #compound #chemicalformula #science #rasayansha. 23.3 structure and properties of amines. Consisting of a phenyl group (−c 6 h 5) attached to an amino group (−nh 2), aniline is the simplest aromatic amine. Explain why most chiral amines cannot be resolved into their two enantiomers. After completing this section, you should be able to. Aniline is substantially less basic than methylamine, as is evident by looking at the pk a values for their respective ammonium conjugate acids (remember that the lower the pka of the conjugate acid, the weaker the base). Anilines are an industrially significant commodity chemical. This problem has been solved!
15.11 Amines Structures and Names Chemwiki

Chapter 17 Amines and Amides Chemistry 114 with Divis at Franciscan
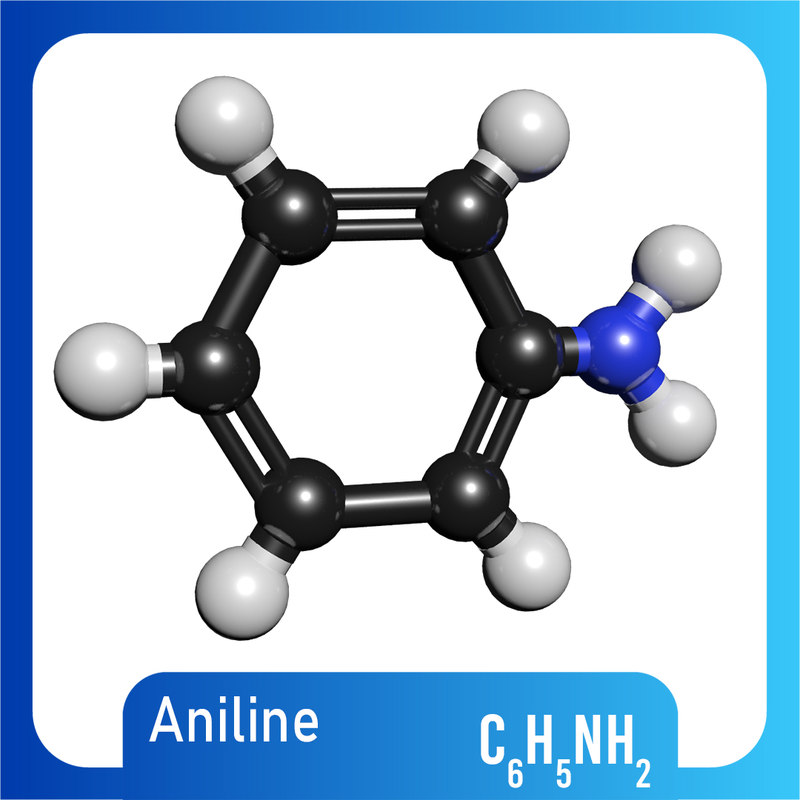
3D c6h5nh2 molecule aniline TurboSquid 1422230
Draw the structure of aniline

Aniline molecule Stock Image F004/5536 Science Photo Library
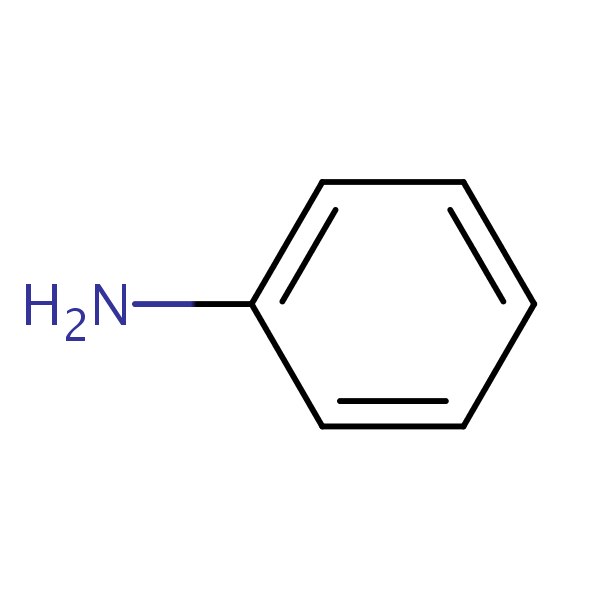
Aniline SIELC

Aniline molecule Royalty Free Vector Image VectorStock
Draw the structure of aniline

Aniline stock vector. Illustration of pigment, paracetamol 42026736
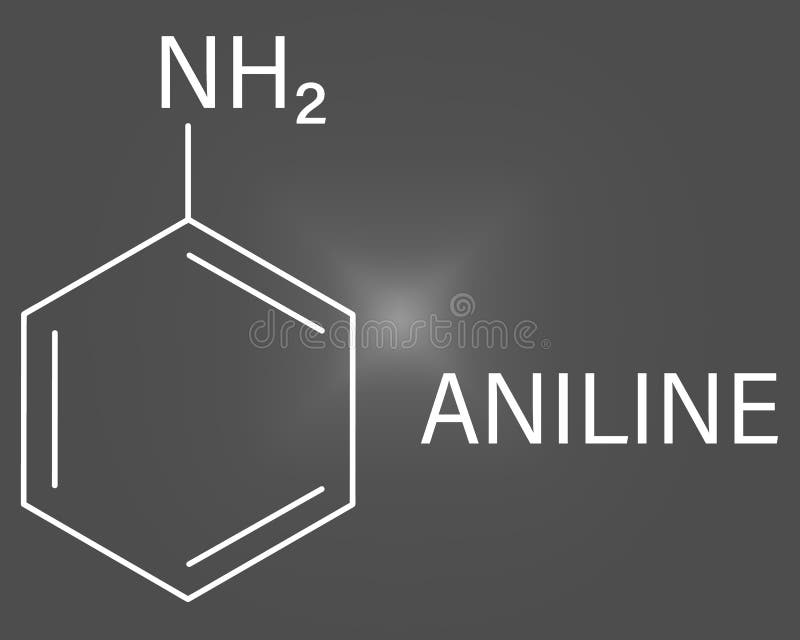
Aniline Molecule. Also Known As Phenylamine, Aminobenzene. Skeletal
Web This Structure Is Also Available As A 2D Mol File Or As A Computed 3D Sd File The 3D Structure May Be Viewed Using Java Or Javascript.
Weast And Grasselli, 1989 Crc.
It Is A Primary Amine Having An Ethyl Group Located Para To The Amino (Nh 2) Group.
Web An Introduction To Phenylamine (Aniline) | Chemkey.
Related Post: