Draw The Structure Of An Atom
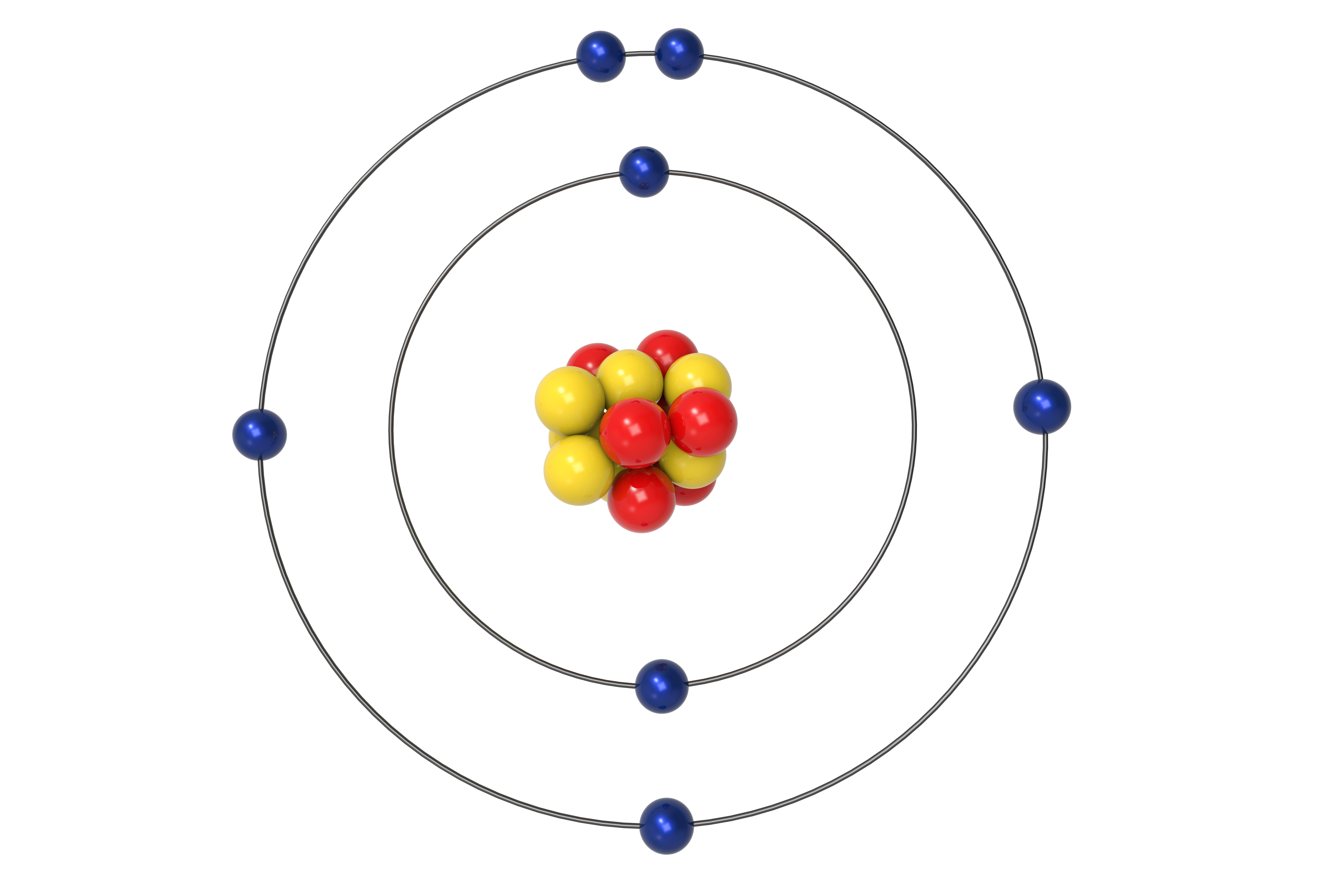
Draw The Structure Of An Atom - The atomic number of an element describes the total number of protons in its nucleus. Most of the atom is empty space. How to draw lewis structures. Discovery of the electron and nucleus. For one thing, dalton considered atoms to. Draw the skeletal structure of the products formed when the given triacylglycerol is hydrolyzed with water in the presence of sodium hydroxide. Drawing atomic structure requires only a simple understanding of the components of atomic structure. The rest consists of three basic types of subatomic particles: Web to draw the lewis structure of an atom, write the symbol of the atom and draw dots around it to represent the valence electrons. Web build an atom out of protons, neutrons, and electrons, and see how the element, charge, and mass change. The structure of the atom. The total number of protons and neutrons in an atom is called its mass number (a). Part of chemistry (single science) atomic structure. Web figure 2.2.1 2.2. Relate electron configurations to element classifications in. Web a neutral atom must contain the same number of positive and negative charges, so the number of protons equals the number of electrons. Also, helium is shown in group 8a, but it only has two valence electrons. Ions are those species which have a positive or a negative charge. Given a chemical formula corresponding to a molecule or molecular. If you understand how protons and electrons relate to one another, as well as how neutrons aid in comprising atomic mass, the rest is cake. Describe the three main subatomic particles. The numbers of subatomic particles in an. Drawing atomic structure requires only a simple understanding of the components of atomic structure. Identify and explain exceptions to predicted electron configurations. Web to draw the lewis structure of an atom, write the symbol of the atom and draw dots around it to represent the valence electrons. The nucleus is positively charged since the proton is positively charged and the neutron is neutral. You will also learn about the properties that can be found through the subatomic particles. The protons and neutrons. The number of protons determines which element is represented, the number of electrons determines its charge, and the number of neutrons determines which isotope of the element is represented. Most of the atom is empty space. Web in an ionic bond, it is more like one atom donating an electron to the other atom. Web do you want to learn. The total number of protons and neutrons in an atom is called its mass number (a). How to draw lewis structures. Part of chemistry (single science) atomic structure. Discovery of the electron and nucleus. The rest consists of three basic types of subatomic particles: Web in an ionic bond, it is more like one atom donating an electron to the other atom. Web how to draw an atom! Atoms have protons and neutrons in the center, making the nucleus, while the electrons orbit the nucleus. Protons and neutrons are found in the nucleus, the dense region at the center of an atom. Then play. Web figure 2.2.1 2.2. Atoms have protons and neutrons in the center, making the nucleus, while the electrons orbit the nucleus. Describe the three main subatomic particles. The modern atomic theory states that atoms of one element are the same, while atoms of different elements are different. See all videos for this article. You may draw the structures in any arrangement that you like, so long as they aren't touching. Discovery of the electron and nucleus. Web science > biology library > chemistry of life > elements and atoms. Describe the three main subatomic particles. Atoms consist of a nucleus containing protons and neutrons, surrounded by electrons in shells. The history of atomic chemistry. Drawing atomic structure requires only a simple understanding of the components of atomic structure. In contrast, electrons are found outside the nucleus in a region called the electron cloud or electron shell. The electrons are arranged in shells around the nucleus. Web primarily, the atomic structure of matter is made up of protons, electrons and. State how the subatomic particles are arranged in atoms. Web updated march 13, 2018. Protons and neutrons reside in the nucleus and are together called nucleons. Describe the three main subatomic particles. Learn how atoms are made up of protons, neutrons, and electrons. Web the classical “planetary” model of an atom. Atoms have protons and neutrons in the center, making the nucleus, while the electrons orbit the nucleus. Elements are defined by the. The rest consists of three basic types of subatomic particles: See all videos for this article. 1) electrons, 2) protons, and neutrons. Web science > biology library > chemistry of life > elements and atoms. The protons and neutrons make up the nucleus of the atom, which is surrounded by the electrons belonging to the atom. Describe the three main subatomic particles. The electrons are arranged in shells around the nucleus. The history of atomic chemistry.
Atom Drawing

Atom Definition, Structure & Parts with Labeled Diagram
Label The Parts Of A Atom
Parts Of An Atom Worksheet

How to draw Atom structure diagram step by step l Atomic structure
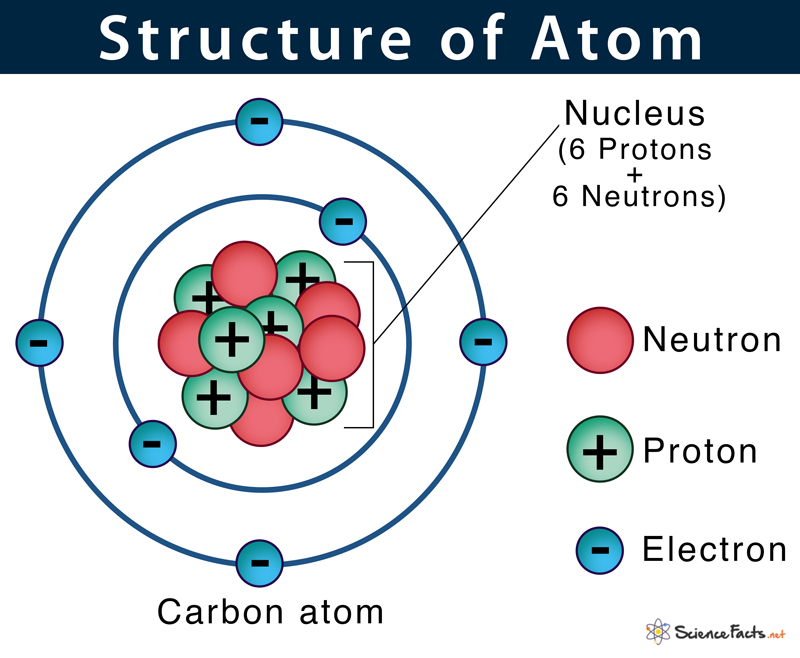
Atom Definition, Structure & Parts with Labeled Diagram
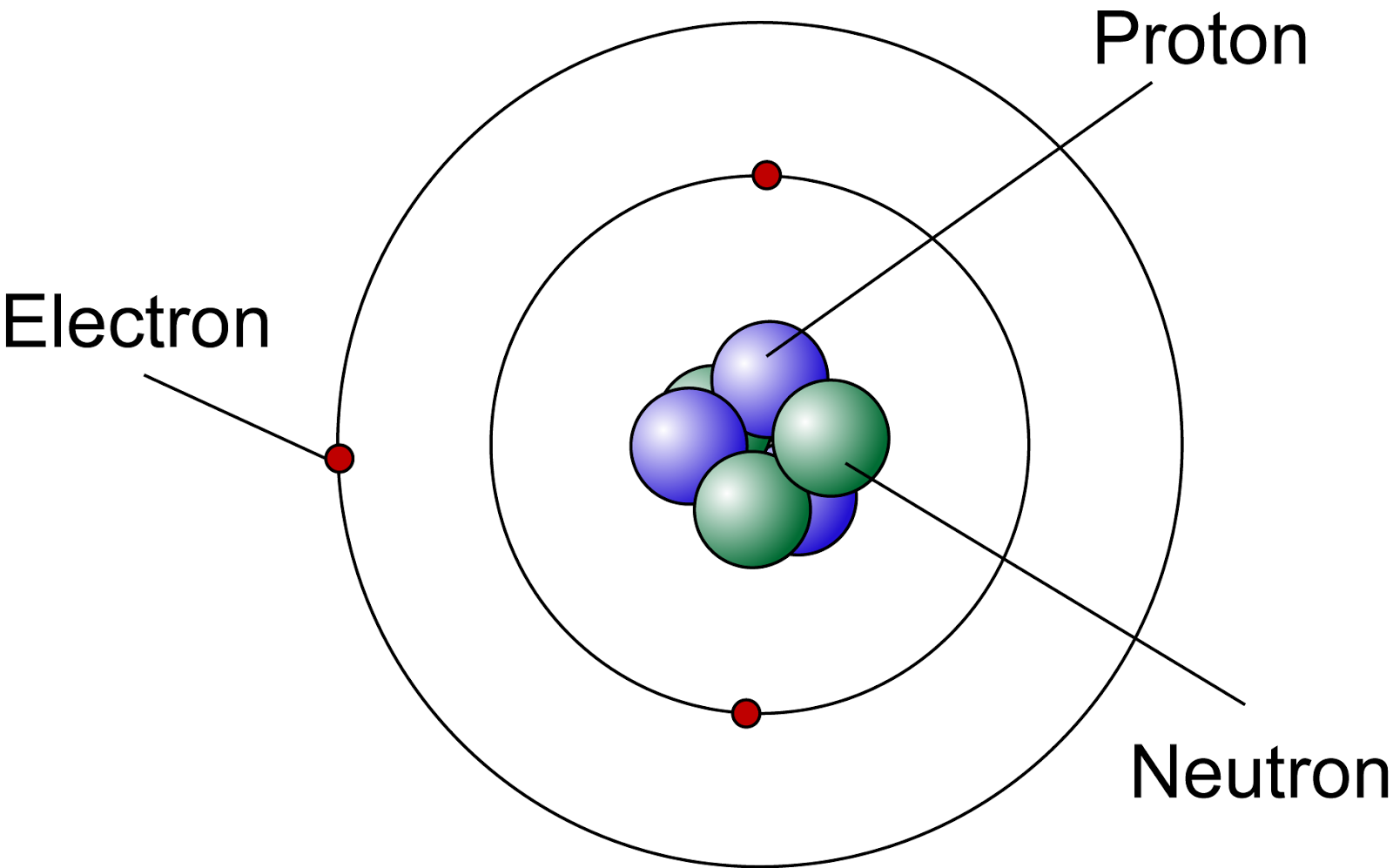
Lets Get Inside An Atom!! The Science Station
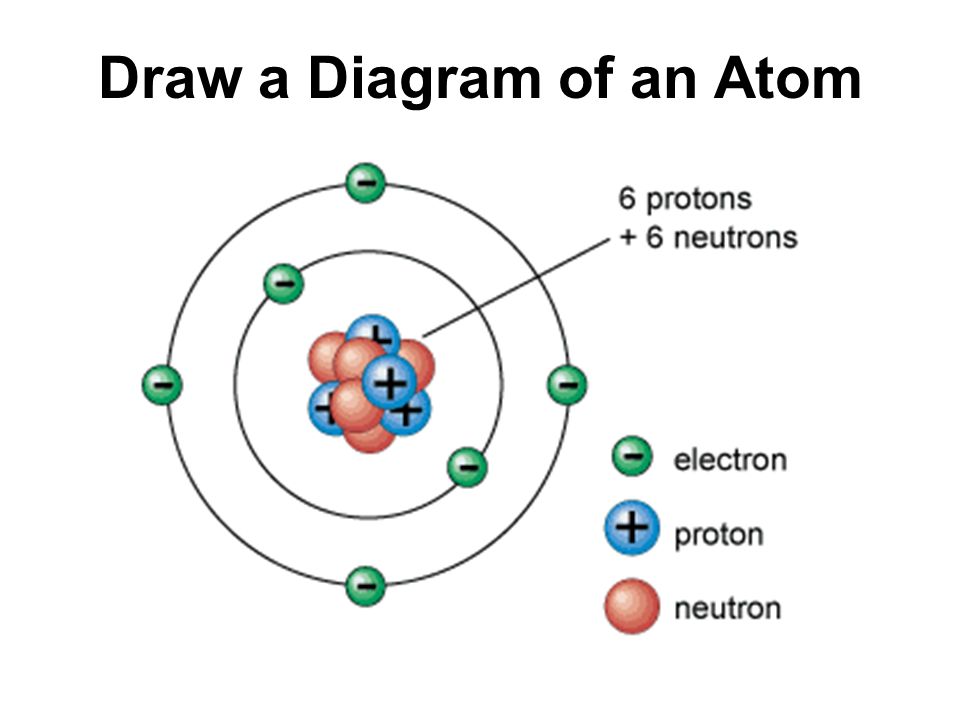
Skills Practice AMAZING 8TH GRADE SCIENTISTS
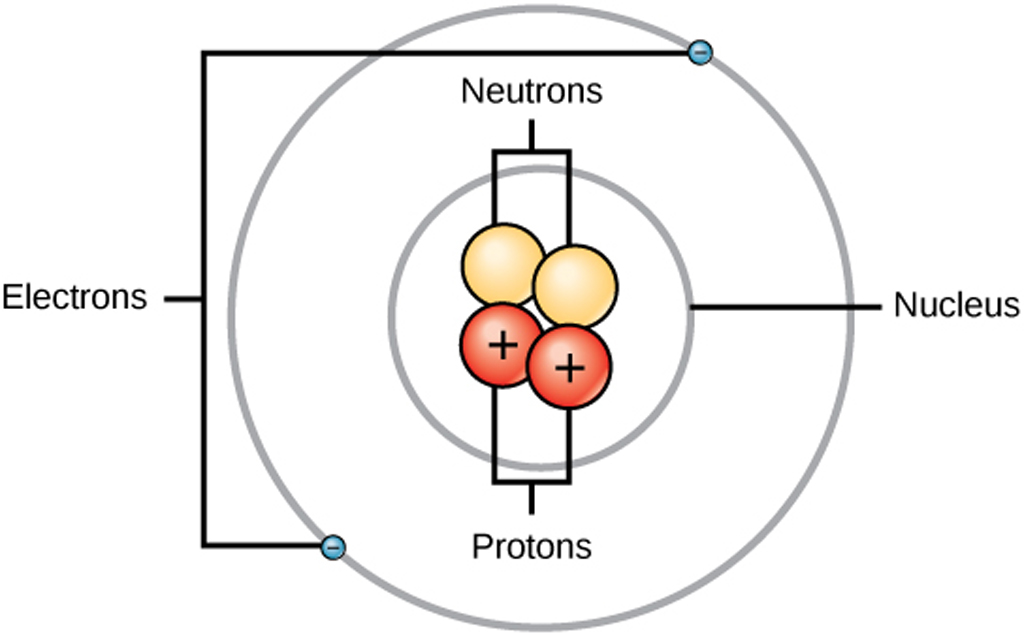
Structure of an Atom Structure & Use of Electron & Proton in Electronics

How to Draw an Atom Really Easy Drawing Tutorial
An Atom Consists Of Three Elementary Subatomic Particles, I.e., Protons, Electrons, And Neutrons.
All Atoms Except Hydrogen Contain Three Basic Subatomic Particles:
There Have Been Several Minor But Important Modifications To Dalton’s Atomic Theory.
Web Figure 2.2.1 2.2.
Related Post: