Draw The Lewis Structure Of Hcn Include Lone Pairs
Draw The Lewis Structure Of Hcn Include Lone Pairs - For the hcn lewis structure, calculate the total number of valence electrons for the hcn. Web write the chemical formulas for compounds with two nitrogen atoms and four oxygen atoms. The lewis structure of hcn shows that the carbon atom is the central atom and is covalently. Hydrogen (h), carbon (c), and nitrogen (n). Beginning with the terminal atoms, add enough electrons to each. Hcn draw the lewis structure of co including lone. Thus far, we have discussed. Understand the proper use of the octet rule to predict bonding in simple molecules. Put least electronegative atom in centre3. Put one electron pair in each bond4. Beginning with the terminal atoms, add enough electrons to each. The lewis structure of hcn shows that the carbon atom is the central atom and is covalently. Understand the proper use of the octet rule to predict bonding in simple molecules. Choose a likely identity for x, y, and z in these structures. Web the lewis structure (lewis dot diagram). Put least electronegative atom in centre3. Beginning with the terminal atoms, add enough electrons to each. Web the lewis structure indicates that each cl atom has three pairs of electrons that are not used in bonding (called lone pairs) and one shared pair of electrons (written between. Web draw the lewis structure of hcn. Draw the lewis structure of the. Web the lewis structure indicates that each cl atom has three pairs of electrons that are not used in bonding (called lone pairs) and one shared pair of electrons (written between. Thus far, we have discussed. Choose a likely identity for x, y, and z in these structures. Draw the lewis structure of the following molecule. Web write the chemical. Put least electronegative atom in centre3. Understand the proper use of the octet rule to predict bonding in simple molecules. Beginning with the terminal atoms, add enough electrons to each. Thus far, we have discussed. Hydrogen (h), carbon (c), and nitrogen (n). Web draw the lewis structure of hcn. Web write the chemical formulas for compounds with two nitrogen atoms and four oxygen atoms. Put least electronegative atom in centre3. Web the lewis structure indicates that each cl atom has three pairs of electrons that are not used in bonding (called lone pairs) and one shared pair of electrons (written between. Put. Web the hcn molecule consists of three atoms: Hcn draw the lewis structure of co including lone. To draw the lewis structure of hcn, place h at the center and connect it to c. Web draw the lewis structure of hcn. Web the lewis structure (lewis dot diagram) for hcn.1. Hydrogen (h), carbon (c), and nitrogen (n). Web in h 2 o, for example, there is a bonding pair of electrons between oxygen and each hydrogen. Web draw the lewis structure of hcn. H single line to c, triple line to n (one set of two dots) change the bond between the two carbon atoms in each molecule. Web the. Beginning with the terminal atoms, add enough electrons to each. Put least electronegative atom in centre3. Hcn draw the lewis structure of co including lone. Put one electron pair in each bond4. H single line to c, triple line to n (one set of two dots) change the bond between the two carbon atoms in each molecule. Thus far, we have discussed. Put one electron pair in each bond4. Web the lewis structure (lewis dot diagram) for hcn.1. Draw the lewis structure of the following molecule. Web in h 2 o, for example, there is a bonding pair of electrons between oxygen and each hydrogen. Draw the lewis structure of the following molecule. For the hcn lewis structure, calculate the total number of valence electrons for the hcn. Choose a likely identity for x, y, and z in these structures. To draw the lewis structure of hcn, place h at the center and connect it to c. Web the lewis structure indicates that each cl. To draw the lewis structure of hcn, place h at the center and connect it to c. Web the lewis structure (lewis dot diagram) for hcn.1. Choose a likely identity for x, y, and z in these structures. Web write the chemical formulas for compounds with two nitrogen atoms and four oxygen atoms. The lewis structure of hcn shows that the carbon atom is the central atom and is covalently. H single line to c, triple line to n (one set of two dots) change the bond between the two carbon atoms in each molecule. Hydrogen (h), carbon (c), and nitrogen (n). Thus far, we have discussed. A central x atom is bonded to. 284k views 10 years ago lewis structures practice problems with answers. Web the lewis structure indicates that each cl atom has three pairs of electrons that are not used in bonding (called lone pairs) and one shared pair of electrons (written between. Add lone pairs around n and c to satisfy the octet rule. Beginning with the terminal atoms, add enough electrons to each. Web draw the lewis structure of hcn. Understand the proper use of the octet rule to predict bonding in simple molecules. Web draw lewis structures depicting the bonding in simple molecules.
Hcn Lewis Structure Bonds Draw Easy
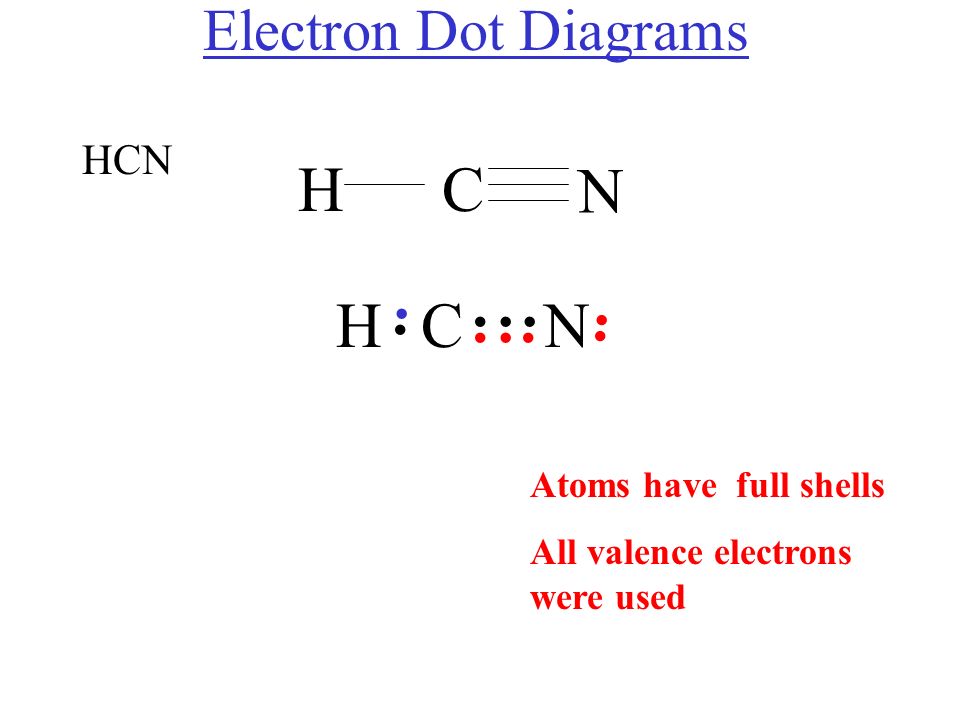
Lewis Diagram For Hcn
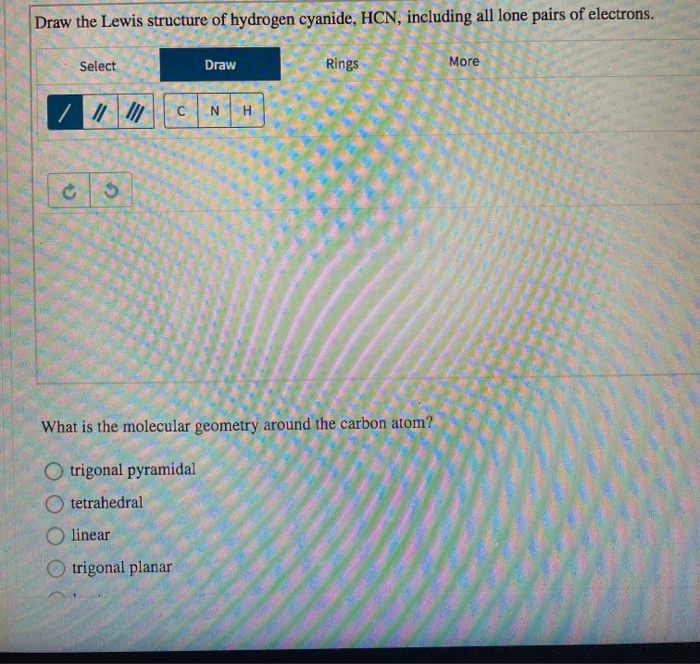
Draw The Lewis Structure Of Hydrogen Cyanide, HCN, Including All Lone

HCN Lewis Structure, Molecular Geometry, Hybridization, MO Diagram, and
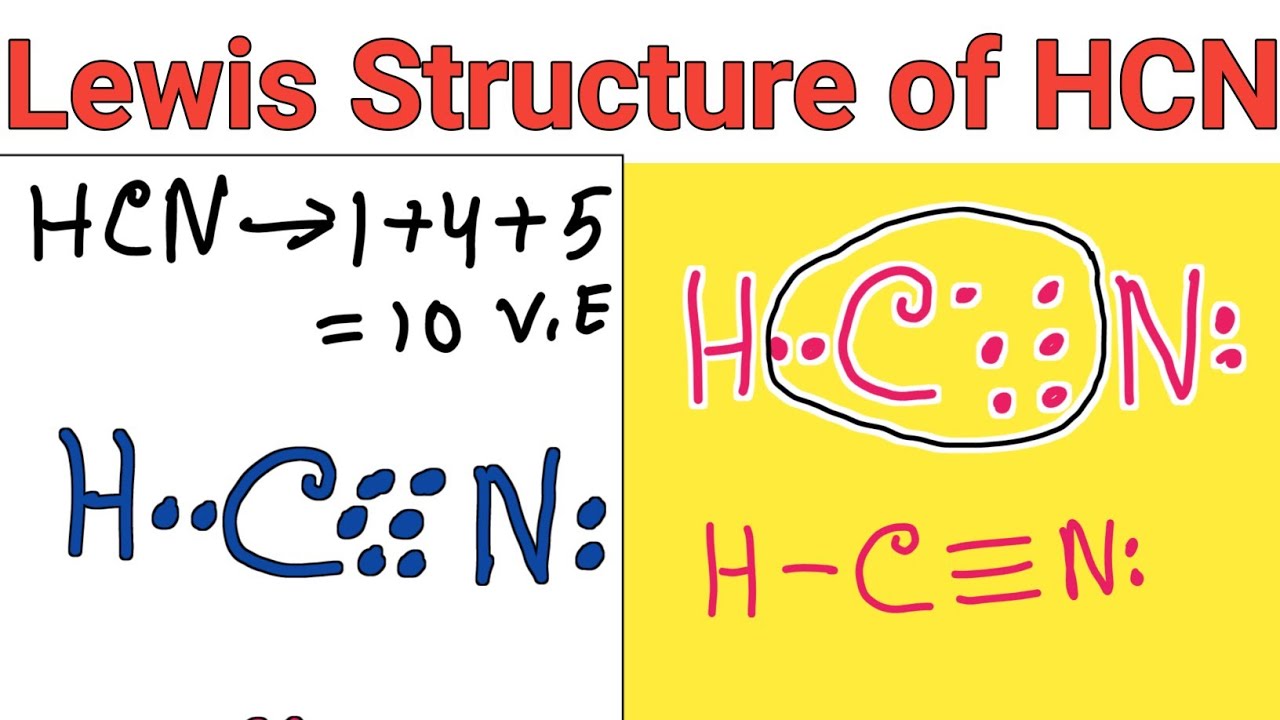
Lewis structure of HCN (Hydrogen cyanide) YouTube

Lewis Dot Diagram Of Hcn
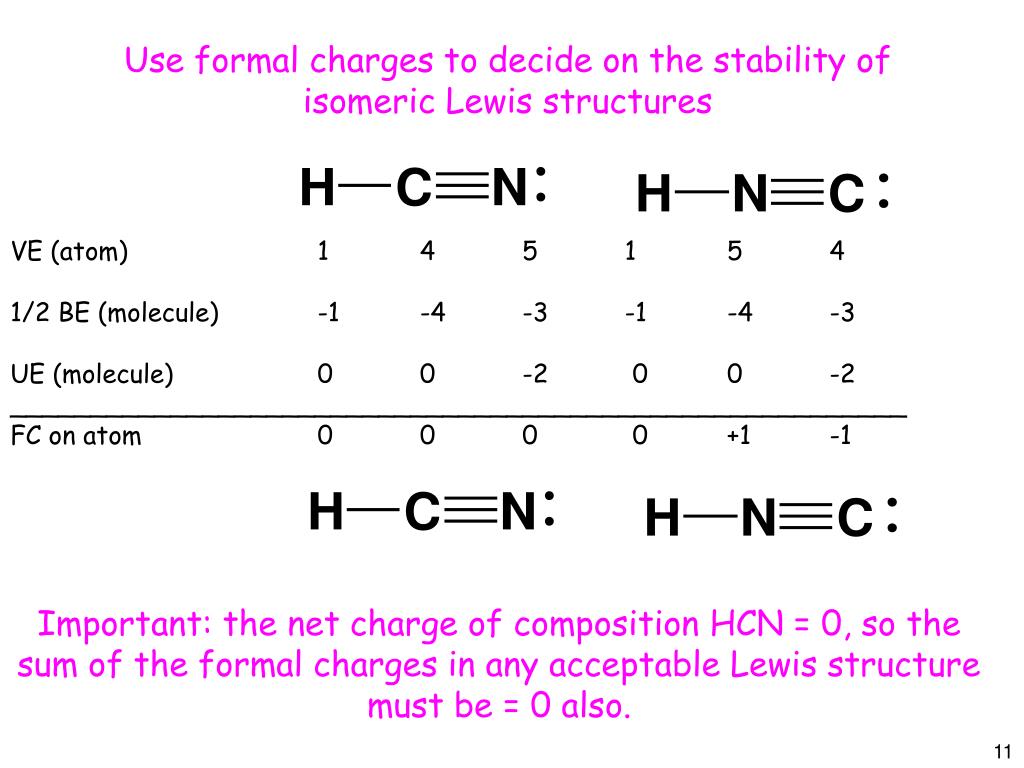
Estrutura De Lewis Hcn
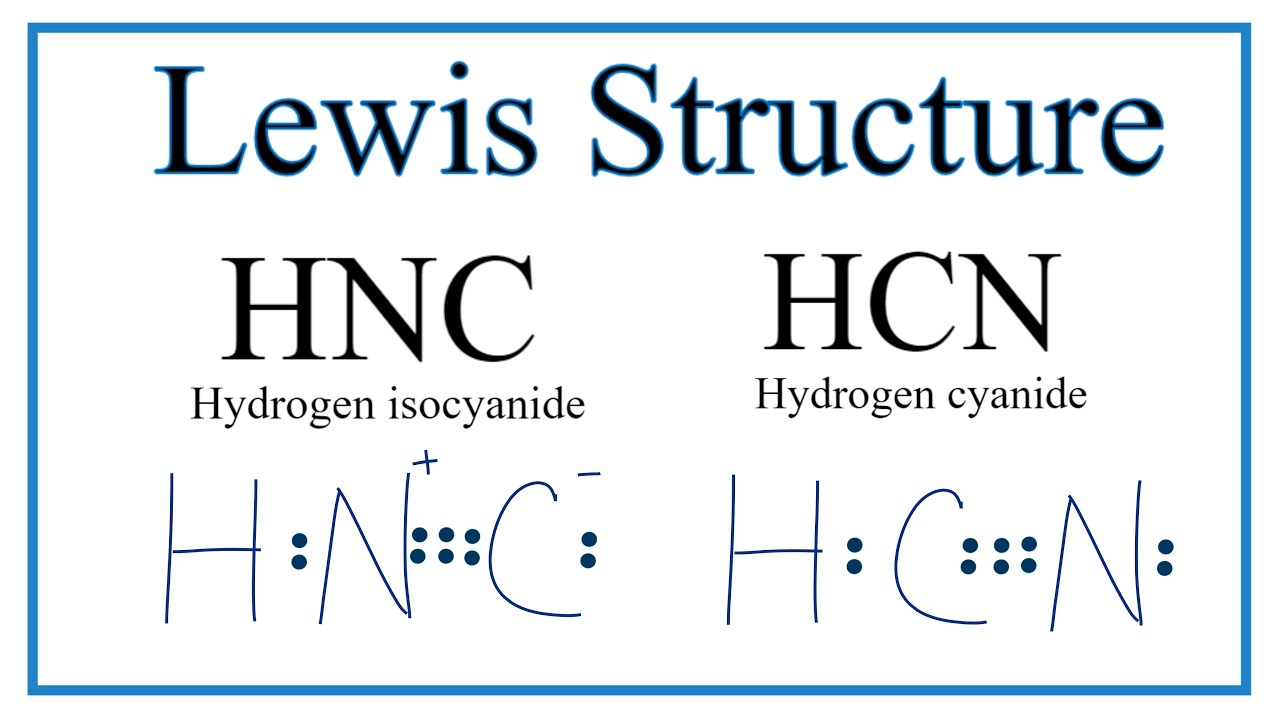
Hcn Lewis Structure Bonds Draw Easy
[Solved] Draw the Lewis structure of HCN. Include lone pairs. Select
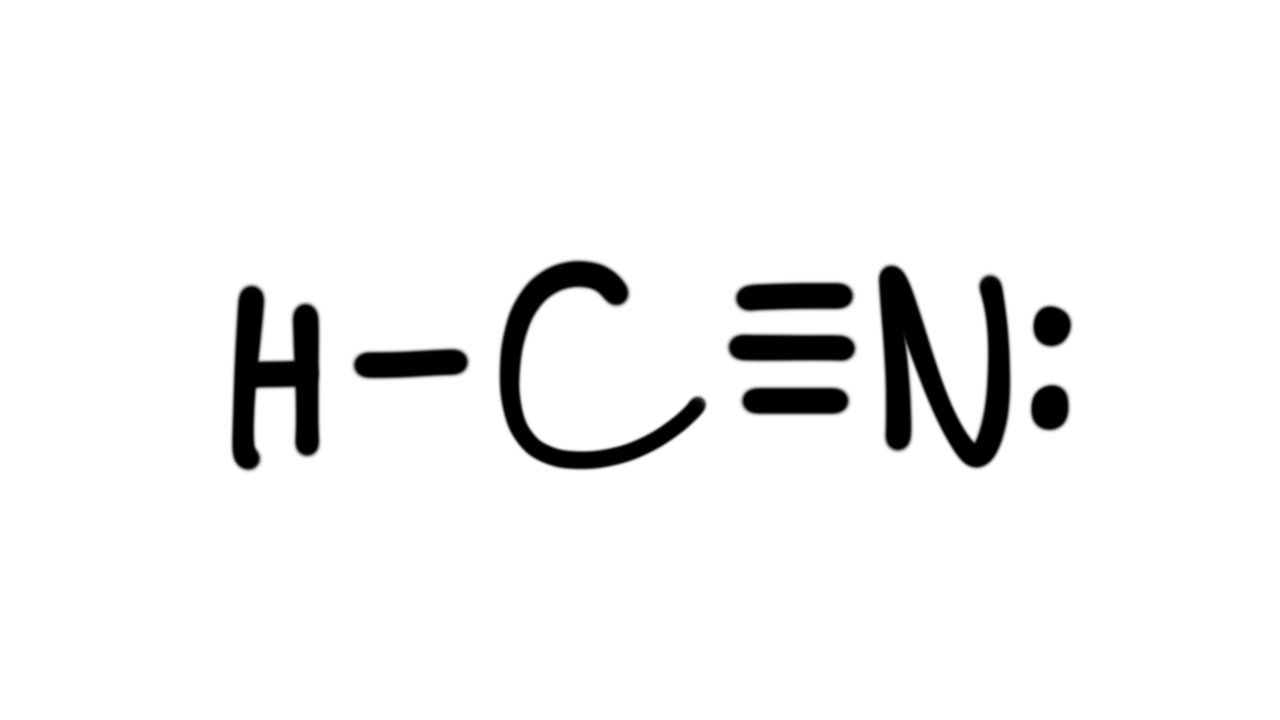
Estrutura De Lewis Hcn
Put One Electron Pair In Each Bond4.
Web The Hcn Molecule Consists Of Three Atoms:
Web In H 2 O, For Example, There Is A Bonding Pair Of Electrons Between Oxygen And Each Hydrogen.
Web The Lewis Structure Indicates That Each Cl Atom Has Three Pairs Of Electrons That Are Not Used In Bonding (Called Lone Pairs) And One Shared Pair Of Electrons (Written Between.
Related Post: