Draw The Lewis Structure For The Sulfur Hexafluoride Molecule
Draw The Lewis Structure For The Sulfur Hexafluoride Molecule - The lewis dot structure of any molecule is a pictorial representation of the atoms involved in forming the structure and its individual valence electrons. The lewis structure of sf6 ( sulfur hexafluoride) consists of a central sulfur atom bonded to six fluorine atoms. 1.4k views 3 years ago lewis structure (chemistry) how to draw a lewis structure for sf6 (sulfur hexafluoride) lewis structure: This problem has been solved! There are three lone pairs on each fluorine atom, and the sulfur atom does not have any lone pair. In this tutorial, we will learn how to draw the lewis structure of sf 4. • how to draw lewis. A video explanation of how to draw the lewis dot structure for sulfur. 4k views 11 years ago chemistry lewis dot structures. The sulfur has six valence electrons, and must make six bonds to form a molecule with the six fluorine atoms. Web lewis dot of sulfur hexafluoride. Sf 6 is a colorless odorless, nontoxic and nonflammable gas. When inhaled it has the opposite effect that helium would have. 100% (10 ratings) share share. A) what is the electron pair geometry? It has a density of 6.1g/l (at sea level), which is a very dense gas. When inhaled it has the opposite effect that helium would have. Each fluorine atom has one valence electron and will make one bond each. In this tutorial, we will learn how to draw the lewis structure of sf 4. (6 points) ch3 en h3c .0: You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Web lewis dot of sulfur hexafluoride. Draw the lewis structure for sulfur hexafluoride (sf6) and answer the following questions: There are no lone pairs on sulfur atom and three lone pairs on each fluorine atom. This problem has been solved! Here’s how to approach this question. 70 more lewis dot structures. Sf 6 is a colorless odorless, nontoxic and nonflammable gas. The lewis structure of sf6 ( sulfur hexafluoride) consists of a central sulfur atom bonded to six fluorine atoms. Each fluorine atom has one valence electron and will make one bond each. It has a density of 6.1g/l (at sea level), which is a very dense gas. 1.4k views 3 years ago lewis structure (chemistry) how to draw a lewis structure for sf6 (sulfur hexafluoride) lewis structure: Thus sf6 has 48 valence electrons that will help us draw the lewis dot structure of sf6. Draw the lewis structure of the sulfur hexafluoride. How to draw the lewis structure for sf6. 70 more lewis dot structures. Draw the lewis structure for sulfur hexafluoride (sf6) and answer the following questions: 1.4k views 3 years ago lewis structure (chemistry) how to draw a lewis structure for sf6 (sulfur hexafluoride) lewis structure: Draw the lewis structure of the sulfur hexafluoride molecular sf, and use the drawing. Web = 6 + 42. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. When inhaled it has the opposite effect that helium would have. Sf 6 is a colorless odorless, nontoxic and nonflammable gas. A video explanation of how to draw the lewis dot structure for sulfur. The sulfur atom has six valence electrons, while each fluorine atom contributes one valence electron. • how to draw lewis. Identify the total number of valence electrons in the sulfur hexafluoride (sf6) molecule. Web in sf 6 lewis structure, each fluorine atom has made single bonds with center sulfur atom. Indicate the hybridization (sp, sp’, sp, spºd, sp?d?) for each. When inhaled it has the opposite effect that helium would have. Web draw the lewis structure for the sulfur hexafluoride (sf6) molecule. (6 points) ch3 en h3c .0: The structure of caffeine is shown. Draw the lewis structure of the sulfur hexafluoride molecular sf, and use the drawing to determine the molecule's geometry. Web draw the lewis structure for the sulfur hexafluoride (sf6) molecule. It has a density of 6.1g/l (at sea level), which is a very dense gas. The sulfur atom has six valence electrons, while each fluorine atom contributes one valence electron. Web in sf 6 lewis structure, each fluorine atom has made single bonds with center sulfur atom. I quickly. 100% (10 ratings) share share. Draw the lewis structure for the sulfur hexafluoride, sf6. This structure is also available as a 2d mol file. This problem has been solved! Indicate the hybridization (sp, sp’, sp, spºd, sp?d?) for each of the atoms indicated. The lewis dot structure of any molecule is a pictorial representation of the atoms involved in forming the structure and its individual valence electrons. The sulfur has six valence electrons, and must make six bonds to form a molecule with the six fluorine atoms. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Web in sf 6 lewis structure, each fluorine atom has made single bonds with center sulfur atom. Valence electrons of sulfur = 6, and valence electrons of fluorine = 7 there are 6 atoms of fluorine in this compound, so the total valence electrons of fluorine here are = 7*6 = 42 valence electrons (valence electrons are the number of electrons present in the outermost shell of an atom). Web lewis dot of sulfur hexafluoride. How many valence electrons surround the central s atom in this structure? In this tutorial, we will learn how to draw the lewis structure of sf 4. Find the total valence electrons in sf6 molecule. Web draw the lewis structures for sulfur hexafluoride (\(\ce{sf6}\)).
SF6 Molecular Geometry,Shape and Bond Angles (Sulphur Hexafluoride
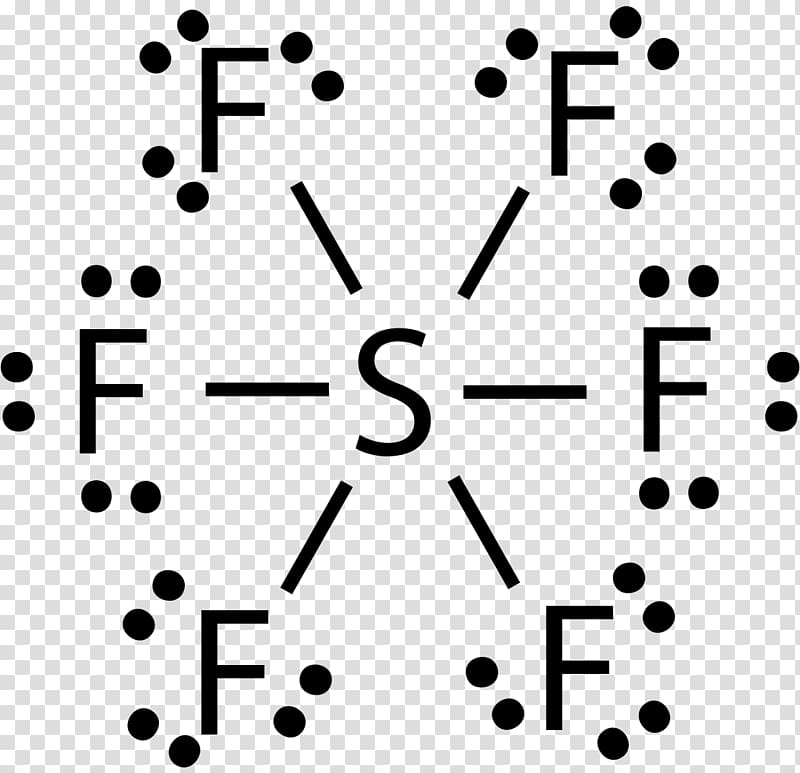
Sulfur Hexafluoride Lewis Structure

Sf6 sulfur hexafluoride molecule Royalty Free Vector Image

Sulfur hexafluoride Electrical Test Tech
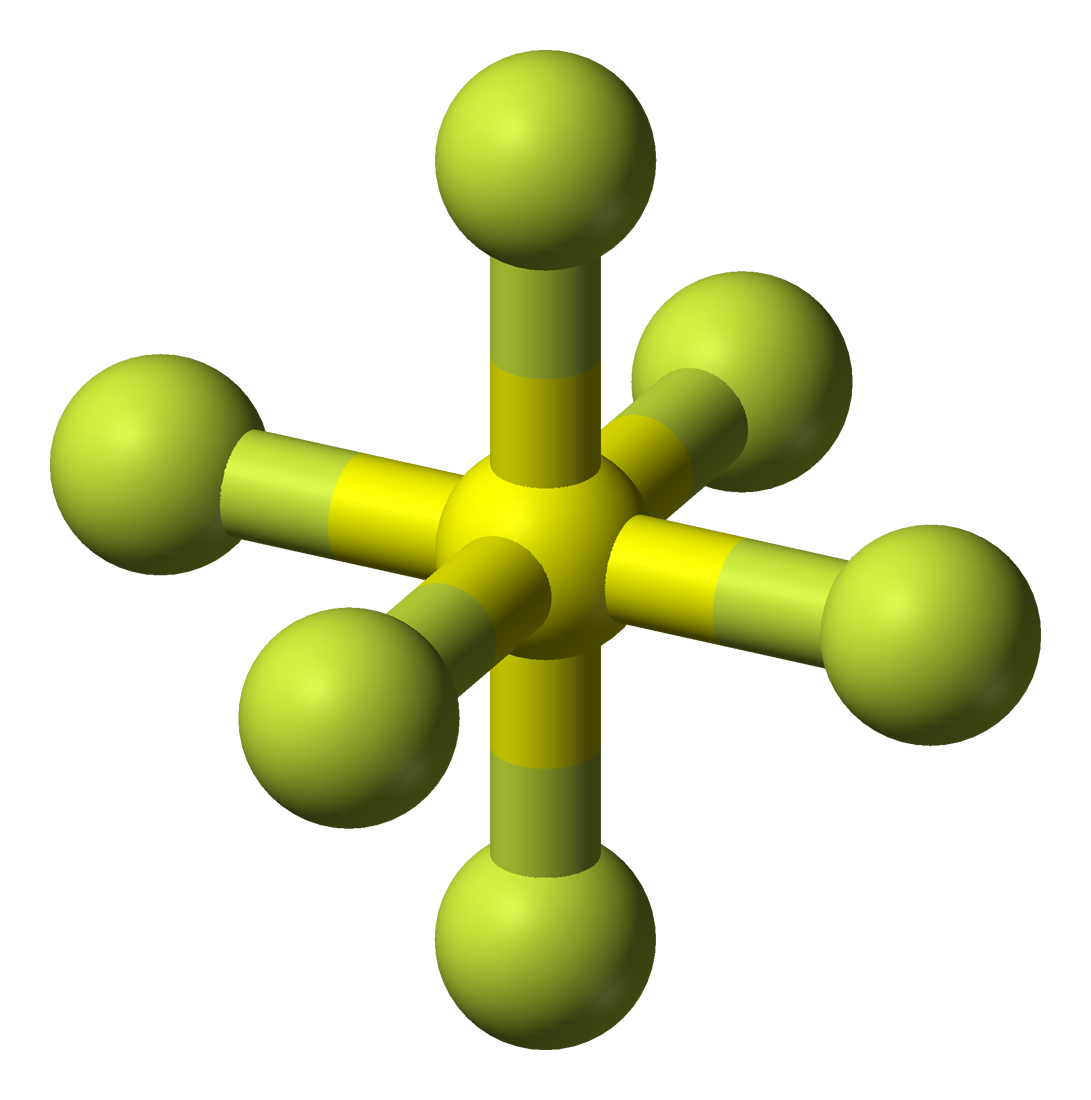
SF6 Molecular Geometry, Lewis Structure, Shape, and Polarity

Sulfur Hexafluoride

Draw the Lewis structure for the sulfur hexafluoride SF6 molecule YouTube
Sulfur Hexafluoride Lewis Structure
Sulfur Hexafluoride Lewis Structure
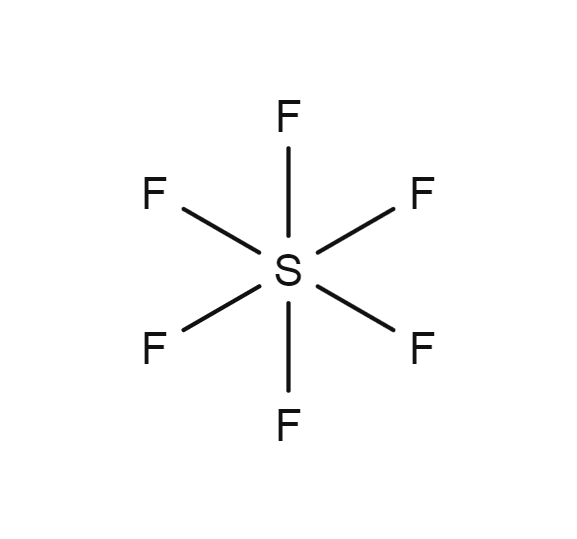
Sulfur hexafluoride Gas Encyclopedia Air Liquide
There Are Three Lone Pairs On Each Fluorine Atom, And The Sulfur Atom Does Not Have Any Lone Pair.
Thus Sf6 Has 48 Valence Electrons That Will Help Us Draw The Lewis Dot Structure Of Sf6.
You'll Get A Detailed Solution From A Subject Matter Expert That Helps You Learn Core Concepts.
This Problem Has Been Solved!
Related Post: