Draw The Lewis Structure For The H3O Ion
Draw The Lewis Structure For The H3O Ion - This is a clip from the complete video:. Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. Web steps of drawing h3o+ lewis structure step 1: How to draw a lewis structure. A) tetrahedral / planar b) tetrahedral / trigonal pyramidal c) planar / planar d) trigonal planar / linear. By the end of this section, you will be able to: H 3 o + lewis structure. Many of the ions that form have eight electrons in their valence shell. Web here are the steps to draw a lewis structure. A video explanation of how to draw the lewis dot structure for the hydronium ion, along with information about the. 12k views 11 years ago chemistry lewis dot structures. H3o+ is a chemical formula for the hydronium ion. Web 6 steps to draw the lewis structure of h3o+ ion step #1: Using lewis structures to show valence electrons. Hydrogen atoms only need 2 valence electrons to have a full outer shell. 243k views 10 years ago. Web the following procedure will give you the correct lewis structure for any molecule or polyatomic ion that has one central atom. To use lewis dot symbols to explain the stoichiometry of a compound. How to draw lewis structure. Do not forget to subscribe! Web draw the lewis structure for the (h3o+)ion. Hydrogen atoms only need 2 valence electrons to have a full outer shell. Let’s break down each step in more detail. How to draw a lewis structure. Web draw lewis structures for ionic compounds. It consists of one hydrogen atom and th.more. Therefore, there is a +1 charge on oxygen atom in the lewis structure of h 3 o + ion. Each hydrogen atom has linked with oxygen atom. The example is for the nitrate ion. Web draw the lewis structure for the (h3o+)ion. Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. Only one lone pair exist on oxygen atom. Drawing a lewis structure is pretty simple! 6.5k views 1 year ago lewis structure. Therefore, there is a +1 charge on oxygen atom in the lewis structure of h 3 o + ion. Craig beals shows how to draw the lewis structure for the hydronium ion. A lewis structure helps us to find out about the structure of the compound, types, and the number of bonds, physical properties, and how the compound interacts with other compounds. Many of the ions that form have eight electrons in their valence shell. 243k views 10 years. Find the total valence electrons in h3o+ ion in order to find the total valence electrons in h3o+ ion, first of all you should know the valence electrons present in oxygen atom as well as hydrogen atom. Web we draw lewis structures to predict: Do not forget to subscribe! Web to properly draw the h 3 o + lewis structure,. Drawing the lewis structure for h 3 o +. Using the periodic table to draw lewis dot structures. Draw the lewis structure of hydronium (h3o+) and then determine its electron domain and molecular geometries. Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. Web here are the steps to draw. This is a clip from the complete video:. A video explanation of how to draw the lewis dot structure for the hydronium ion, along with information about the. H 3 o + lewis structure. A) tetrahedral / planar b) tetrahedral / trigonal pyramidal c) planar / planar d) trigonal planar / linear. Drawing the lewis structure for h3o+. Web draw lewis structures for ionic compounds. Hello guys!h3o+ is a chemical. 12k views 11 years ago chemistry lewis dot structures. #4 calculate formal charge and check stability (if octet is already completed on central atom) let’s one by one discuss each step in detail. Let’s break down each step in more detail. Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. Therefore, there is a +1 charge on oxygen atom in the lewis structure of h 3 o + ion. Draw lewis structures depicting the bonding in simple molecules. Web i quickly take you through how to draw the lewis structure of hydronium ion, h3o+. Web the following procedure will give you the correct lewis structure for any molecule or polyatomic ion that has one central atom. 255 views 2 years ago. Web the attached image below shows the lewis structure of hydronium ion; Web here are the steps to draw a lewis structure. A video explanation of how to draw the lewis dot structure for the hydronium ion, along with information about the. A lewis structure is a diagram that shows the chemical bonds between atoms in a molecule and the valence electrons or lone pairs of electrons. Draw the lewis structure of hydronium (h3o+) and then determine its electron domain and molecular geometries. Web draw the lewis structure for the (h3o+)ion. Only one lone pair exist on oxygen atom. The example is for the nitrate ion. Hydrogen atoms only need 2 valence electrons to have a full outer shell. The astute reader may have noticed something:
A stepbystep explanation of how to draw the H3O+ Lewis Structure

What is the Lewis structure of hydronium ion? Quizlet

How to Draw The Lewis Structure of The Hydronium Ion (H3O+) YouTube

Hydronium Lewis Structure

H3o Lewis Structure Molecular Geometry
Draw the structure of the stable positive ion formed when an acid

H3O+ Lewis Structure (Hydronium Ion) YouTube

H3o+ Ion Lewis Structure Draw Easy

H3O+ Lewis Structure Lewis Dot Structure for H3O+ Hydronium ion
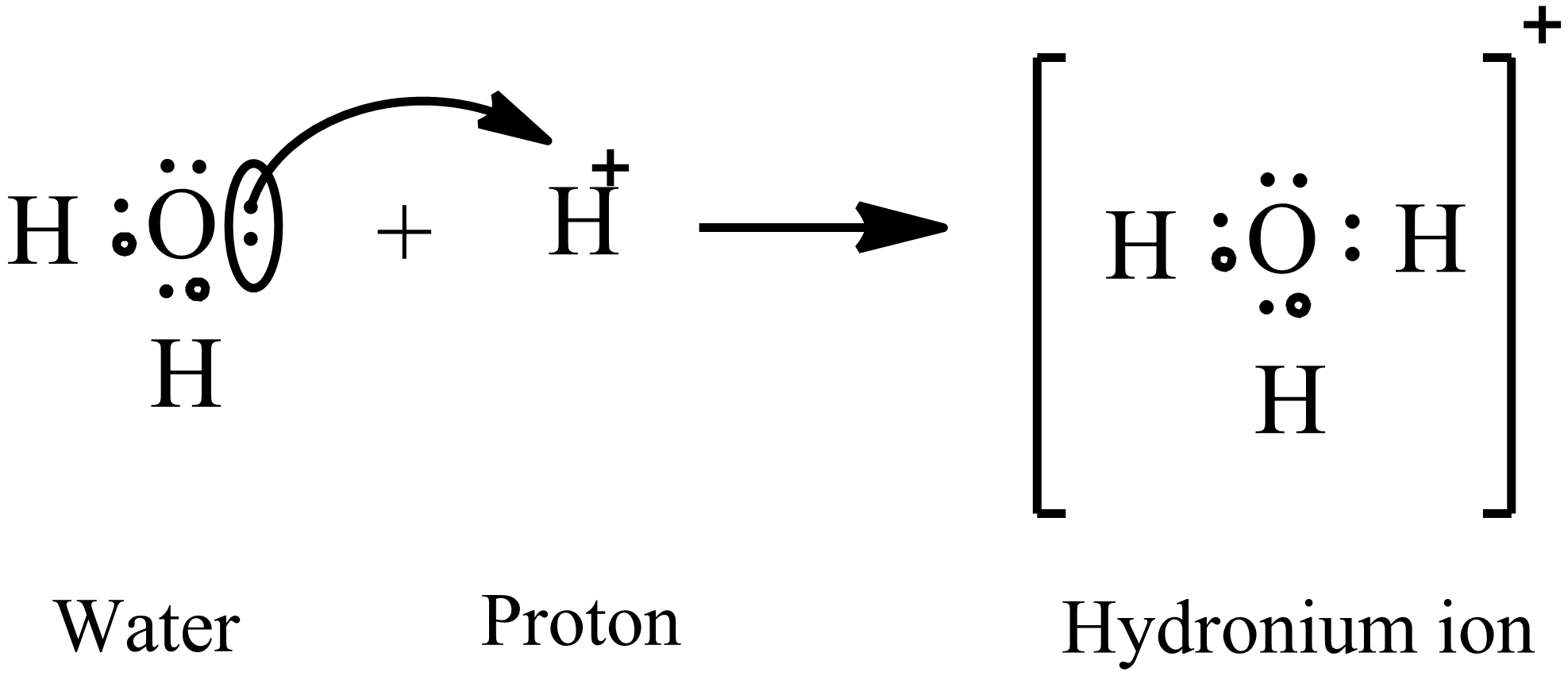
Hydronium Lewis Structure
You'll Get A Detailed Solution From A Subject Matter Expert That Helps You Learn Core Concepts.
There Is +1 Charge And One Lone Pair On Oxygen Atom In H 3 O + Lewis Structure.
Web Steps Of Drawing H3O+ Lewis Structure Step 1:
Here, The Given Ion Is H3O+ Ion (Hydronium Ion).
Related Post:
