Draw The Lewis Structure For The Chlorine Trifluoride Molecule
Draw The Lewis Structure For The Chlorine Trifluoride Molecule - Clf3 lewis structure, molecular structure, hybridization, bond angle and shape. Thus far in this chapter, we have discussed the various types of bonds that form between atoms and/or ions. Chlorine trifluoride or clf3 is an extremely reactive chemical compound with several varied applications and unique physical and chemical compounds. For the clf3 structure use the periodic. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. The chemical formula clf3 represents chlorine trifluoride. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Begin by determining the total number of valence electrons in clf3. The compound is highly reactive, poisonous, and corrosive. Web to draw lewis structures for molecules and polyatomic ions with one central atom. The chemical formula clf3 represents chlorine trifluoride. D) trigonal / square planar. Find the total valence electrons for clf3. Web in this guide, we will take you through the process of drawing the lewis structure for clf3 (chlorine trifluoride), a molecule of importance in various chemical contexts. This problem has been solved! Begin by determining the total number of valence electrons in clf3. • how to draw lewis structure for pocl3. Draw lewis structures depicting the bonding in simple molecules. All atoms are halogens and each has seven valence electrons. Chlorine trifluoride or clf3 is an extremely reactive chemical compound with several varied applications and unique physical and chemical compounds. How to draw lewis structure for clf3 chlorine trifluoride lewis structure: The valence electrons are the electrons in the. D) trigonal / square planar. Despite being famous for its extreme oxidation properties and igniting many things, chlorine. 44k views 11 years ago chemistry lewis dot structures. Thus far in this chapter, we have discussed the various types of bonds that form between atoms and/or ions. Web to draw lewis structures for molecules and polyatomic ions with one central atom. Web draw the lewis structure for the chlorine trifluoride (clf3) molecule. How to draw lewis structure for clf3 chlorine trifluoride lewis structure: A) trigonal bipyramidal / pentagonal. Web draw the lewis structure for the chlorine trifluoride (clf3) molecule. For the clf3 structure use the periodic. Despite being famous for its extreme oxidation properties and igniting many things, chlorine. Web in this guide, we will take you through the process of drawing the lewis structure for clf3 (chlorine trifluoride), a molecule of importance in various chemical contexts. To. Clf3 lewis structure, molecular structure, hybridization, bond angle and shape. A lewis structure is a way to show how atoms share electrons when they form a molecule. The chemical formula clf3 represents chlorine trifluoride. 168k views 10 years ago. Interhalogens are more reactive than pure halogens. However fluorine cannot have more than eight valence electrons in its valence because it is in the second row. It is an interhalogen compound. Lewis structures show all of the valence electrons in an atom or molecule. A lewis structure is a way to show how atoms share electrons when they form a molecule. Then, the electrons are placed around. Thus far in this chapter, we have discussed the various types of bonds that form between atoms and/or ions. Draw the most reasonable lewis structure for the molecule clf3 (chlorine trifluoride). Calculate the total number of valence electrons. Chlorine trifluoride or clf3 is an extremely reactive chemical compound with several varied applications and unique physical and chemical compounds. Draw lewis. Web draw the structures of the following molecules: Vibrational and/or electronic energy levels. Chlorine is capable of hypervalency because it is in the third row of the periodic table; How to draw lewis structure for clf3 chlorine trifluoride lewis structure: Draw the most reasonable lewis structure for the molecule clf3 (chlorine trifluoride). Then, the electrons are placed around the atoms to satisfy the octet rule. For the clf3 structure use the periodic. All atoms are halogens and each has seven valence electrons. It is an interhalogen compound. D) trigonal / square planar. D) trigonal / square planar. Find the total valence electrons for clf3. Web in this guide, we will take you through the process of drawing the lewis structure for clf3 (chlorine trifluoride), a molecule of importance in various chemical contexts. • how to draw lewis structure for pocl3. How to draw lewis structure for clf3 chlorine trifluoride lewis structure: A lewis structure is a way to show how atoms share electrons when they form a molecule. Despite being famous for its extreme oxidation properties and igniting many things, chlorine. To draw the clf3 lewis structure, the valence electrons of the chlorine and fluorine atoms are first counted. Web draw the lewis structure of c l f 3, chlorine trifluoride, ( with minimized formal charges) and then determine its electron domain and molecular geometries. This problem has been solved! Web to draw lewis structures for molecules and polyatomic ions with one central atom. Draw lewis structures depicting the bonding in simple molecules. Gas phase ion energetics data. Web draw the lewis structure for the chlorine trifluoride (clf3) molecule. Web draw the lewis structure for chlorine trifluoride (\(\ce{clf3}\). In order to draw the lewis structure of clf3, first of all you have to find the total number of valence electrons present in the clf3 molecule.
Draw structures of (a) Chlorine trifluoride (b) Chlorine pentafluoride

ClF3 (Chlorine trifluoride) Molecular Geometry, Bond Angles,& Electron

How to Find the Valence Electrons for ClF3 (Chlorine Trifluoride)?

Chf3 Lewis Structure
Molecular Geometry CK12 Foundation

Draw structures of (a) Chlorine trifluoride (b) Chlorine pentafluoride

CLF3 Lewis Structure, Molecular Geometry, and Polarity What's Insight

ClF3 Lewis Structure (Chlorine Trifluoride) YouTube
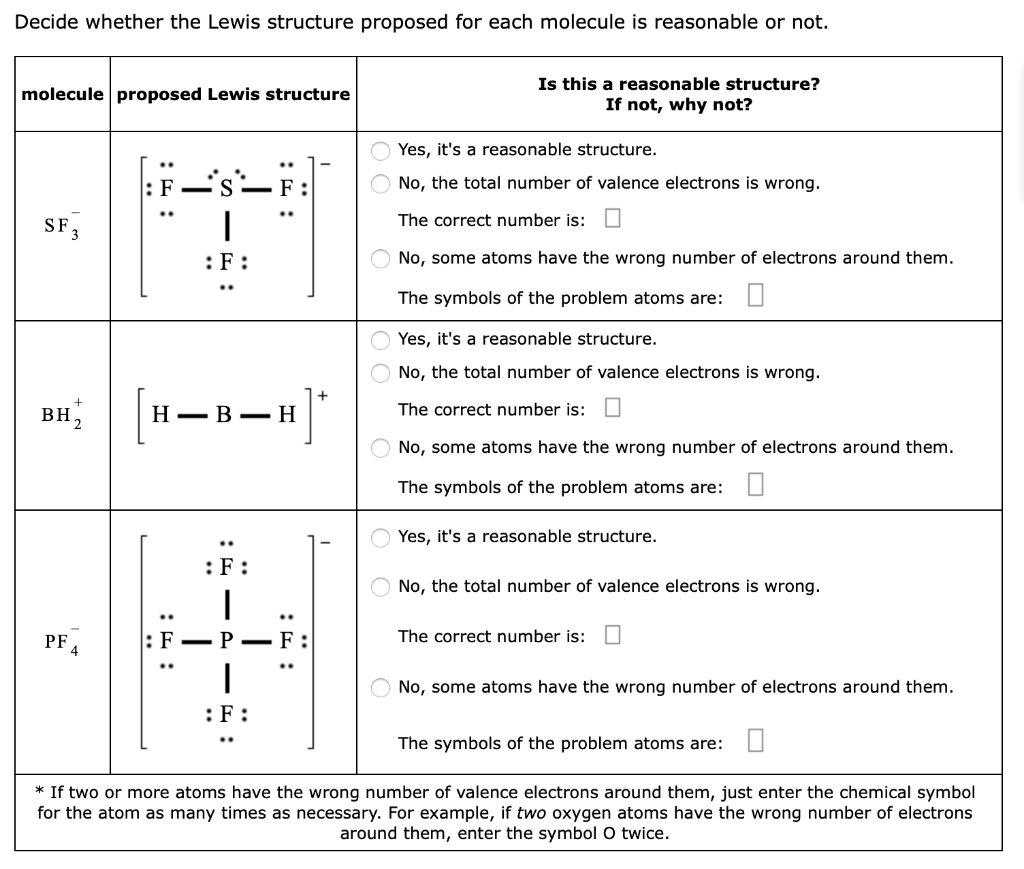
Lewis Structures
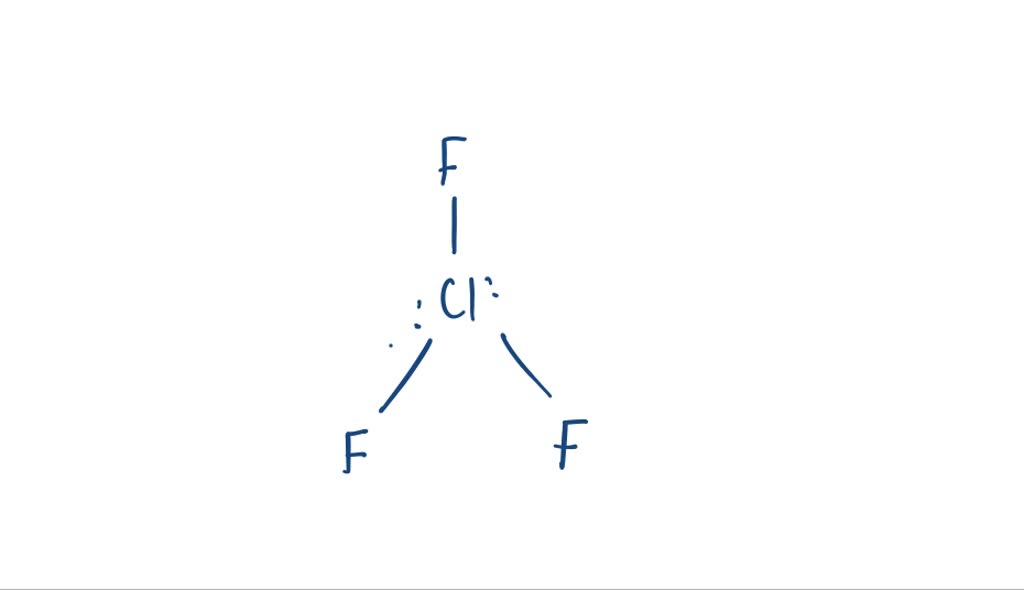
SOLVEDChlorine trifluoride, CIF3, is one of the most reactive
Vibrational And/Or Electronic Energy Levels.
This Problem Has Been Solved!
Begin By Determining The Total Number Of Valence Electrons In Clf3.
By The End Of This Section, You Will Be Able To:
Related Post:
