Draw The Lewis Structure For The Ammonia Molecule
Draw The Lewis Structure For The Ammonia Molecule - Steps of drawing nh3 lewis structure. 1 nitrogen atom needs 3 electrons and all 3 hydrogen atoms need 1 more electron to get stable. Write lewis symbols for neutral atoms and ions. Web this chemistry video tutorial explains how to draw the lewis structure of nh3 also known as ammonia.how to draw lewis structures: Lewis structure of nh3 can be drawn by using valence electrons of nitrogen and hydrogen atoms. Here, the given molecule is nh3 (ammonia). Draw lewis structures depicting the bonding in simple molecules. Find the total valence electrons in nh3 molecule. The nitrogen atom in ammonia has five valence electrons, and the hydrogen. Web 2n2 + 3h2 = 2nh3. Be sure to include all resonance structures that satisfy the octet rule. Steps of drawing nh3 lewis structure. To draw the lewis structure of ammonia, the atom with the least electronegative value is considered. Web this chemistry video tutorial explains how to draw the lewis structure of nh3 also known as ammonia.how to draw lewis structures: Lewis structures show all. To begin drawing the nh3 lewis structure, start by counting the total number of valence electrons. In different fertilizers, ammonia can be used, and it is a good source of nitrogen as well. Valence electrons are the outermost electrons of an atom and are involved in bonding. Web ammonia lewis structure contains three sigma bonds and one lone pair on. By the end of this section, you will be able to: The nitrogen atom in ammonia has five valence electrons, and the hydrogen. Drawing the lewis structure for nh3. Draw lewis structures depicting the bonding in simple molecules. Valence electrons of nh3 ( ammonia ) nh3 lewis structure. Steps of drawing the lewis structure of nh3 is explained in detail in this tutorial. Web to draw the lewis dot diagram of ammonia, we need to understand the valence electron configuration of each atom and the bonding pattern in the molecule. Exceptions to the octet rule; Lewis structures show all of the valence electrons in an atom or molecule.. Craig beals shows how to draw the lewis structure for ammonia. To draw the lewis dot or lewis structure of a molecule is a very challenging as well as an important task. Writing the lewis structures for a molecule with resonance draw the lewis structure for the ammonia (nh,) molecule. In contrast, hydrogen is a group 1 element and only. A lewis structure is a way to show how atoms share electrons when they form a molecule. Valence electrons of nh3 ( ammonia ) nitrogen is a group 15 element and has five electrons in its outer shell. There are 8 valence electrons available for the lewis structure for nh 3. Craig beals shows how to draw the lewis structure. Be sure to include all resonance structures that satisfy the octet rule. Thus far in this chapter, we have discussed the various types of bonds that form between atoms and/or ions. In order to find the total valence electrons in nh3 molecule, first of all you should know the valence electrons present in nitrogen atom as well as hydrogen atom.. Steps of drawing nh3 lewis structure. 6 steps to draw the lewis structure of nh3. Web 2n2 + 3h2 = 2nh3. It also is a good example of a molecule with a trigonal prymidal molecular geometry. Write lewis symbols for neutral atoms and ions. It also provides insights into the hybridization and polarity of ammonia. 6 steps to draw the lewis structure of nh3. It also is a good example of a molecule with a trigonal prymidal molecular geometry. Drawing the lewis structure for nh3. Web chemistry questions and answers. A lewis structure is a way to show how atoms share electrons when they form a molecule. This problem has been solved! Web understanding the lewis structure of ammonia is important as it helps us determine the arrangement of electrons and the geometry of the molecule. 1 nitrogen atom needs 3 electrons and all 3 hydrogen atoms need 1 more. In order to find the total valence electrons in nh3 molecule, first of all you should know the valence electrons present in nitrogen atom as well as hydrogen atom. View the full answer step 2. To draw the lewis dot or lewis structure of a molecule is a very challenging as well as an important task. Draw lewis structures depicting the bonding in simple molecules. 3.8k views 4 years ago lewis structure (chemistry) in this video, you will learn how to draw the lewis structure for chemicals based on total. Lewis structure of nh3 can be drawn by using valence electrons of nitrogen and hydrogen atoms. Web 2n2 + 3h2 = 2nh3. Valence electrons of nh3 ( ammonia ) nitrogen is a group 15 element and has five electrons in its outer shell. Web find how many electrons are required in total: By the end of this section, you will be able to: Valence electrons of nh3 ( ammonia ) nh3 lewis structure. This will help you understand the molecule’s electronic structure and bonding. Web ammonia lewis structure contains three sigma bonds and one lone pair on nitrogen atom. Craig beals shows how to draw the lewis structure for ammonia. This is a clip from the complete video: This problem has been solved!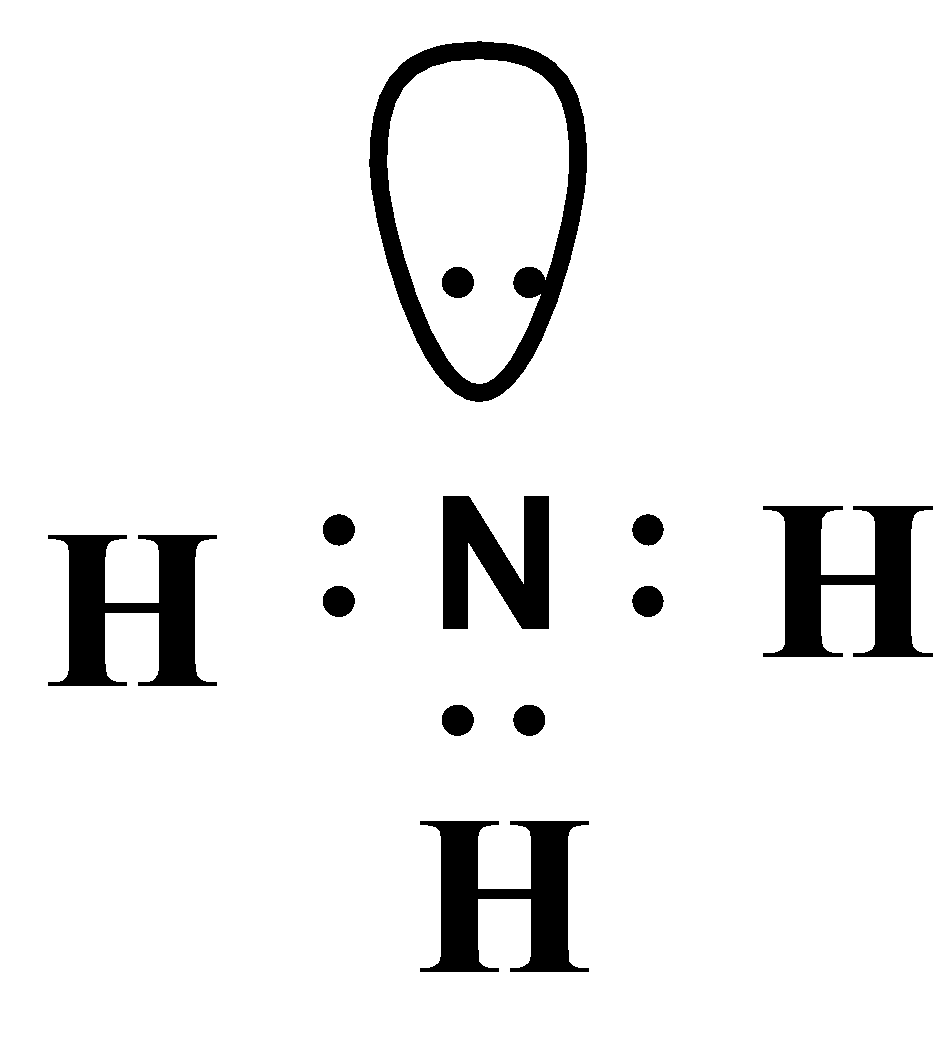
Lewis Dot Structure For Ammonia
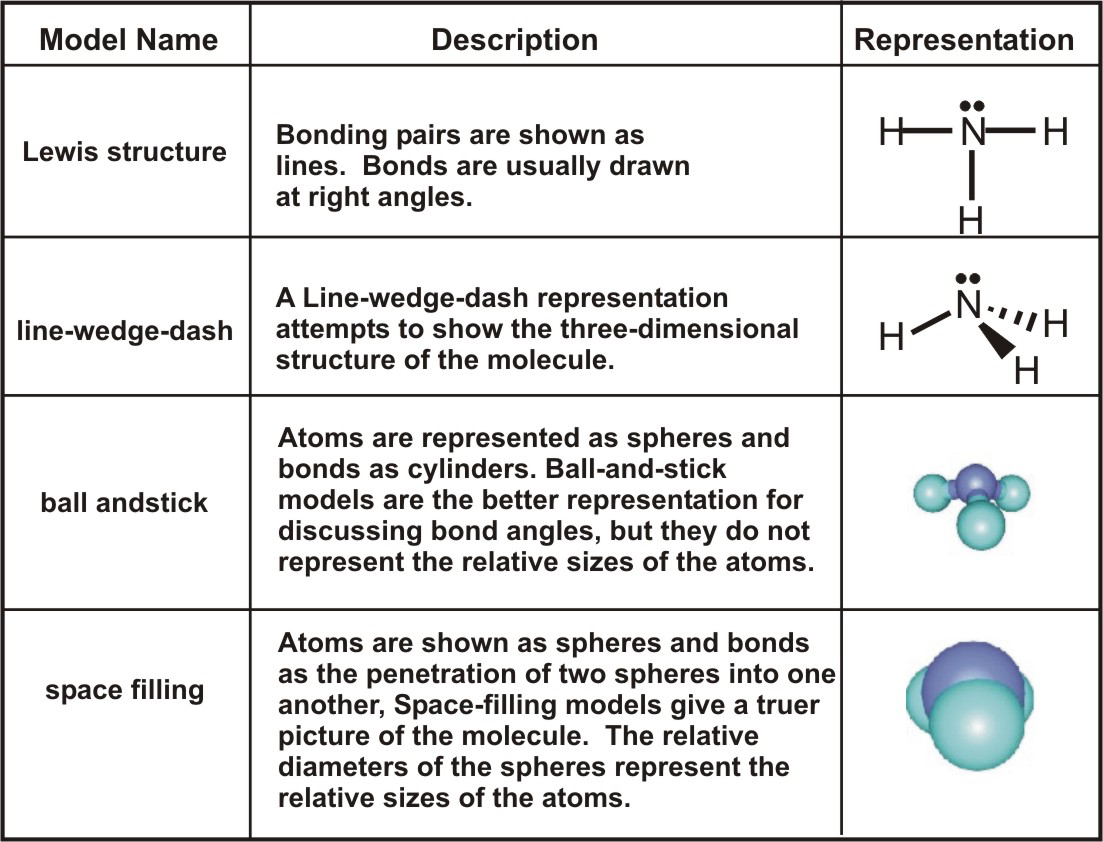
Chapter 6 Molecular Structure
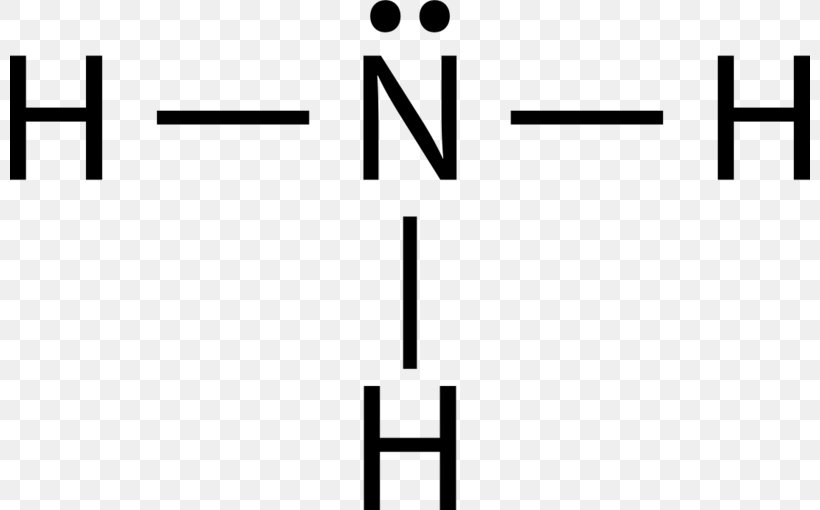
How To Draw The Lewis Structure For Ammonia Science E vrogue.co
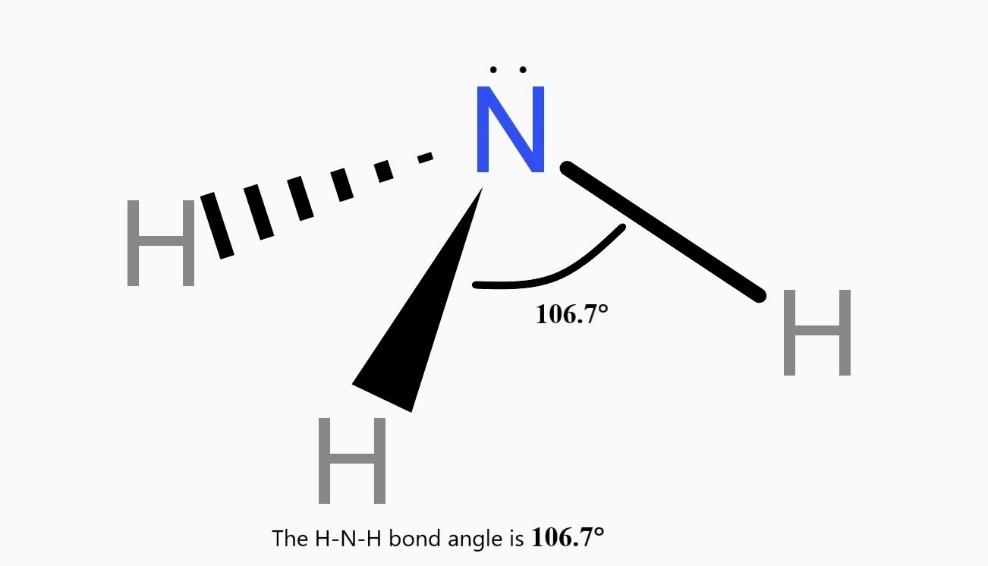
Ammonia Molecule Structure
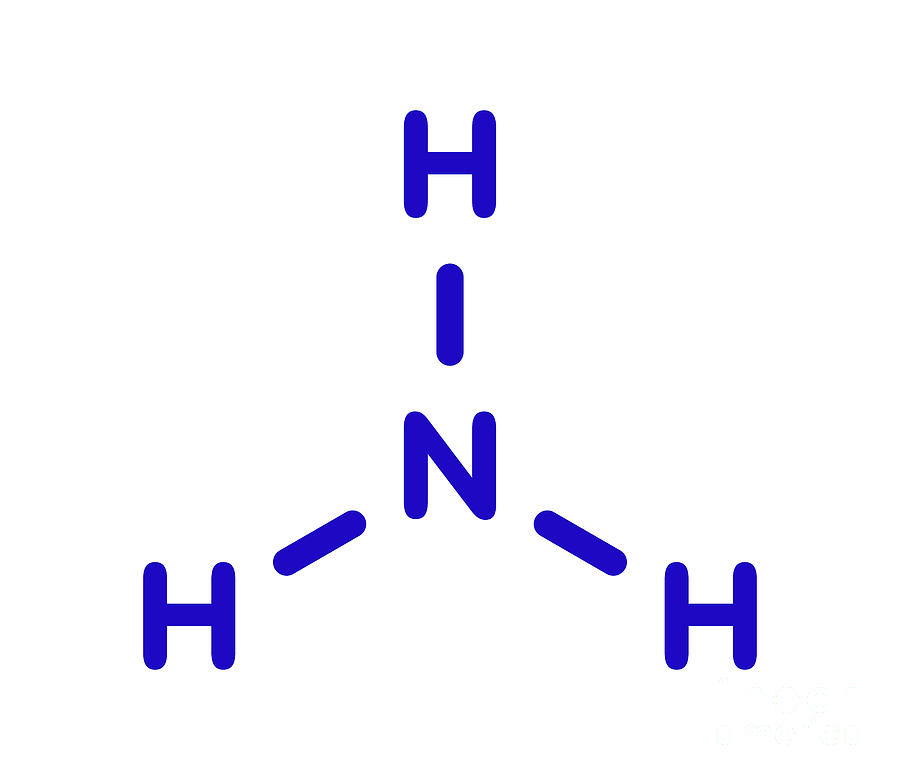
Ammonia Molecular Structure
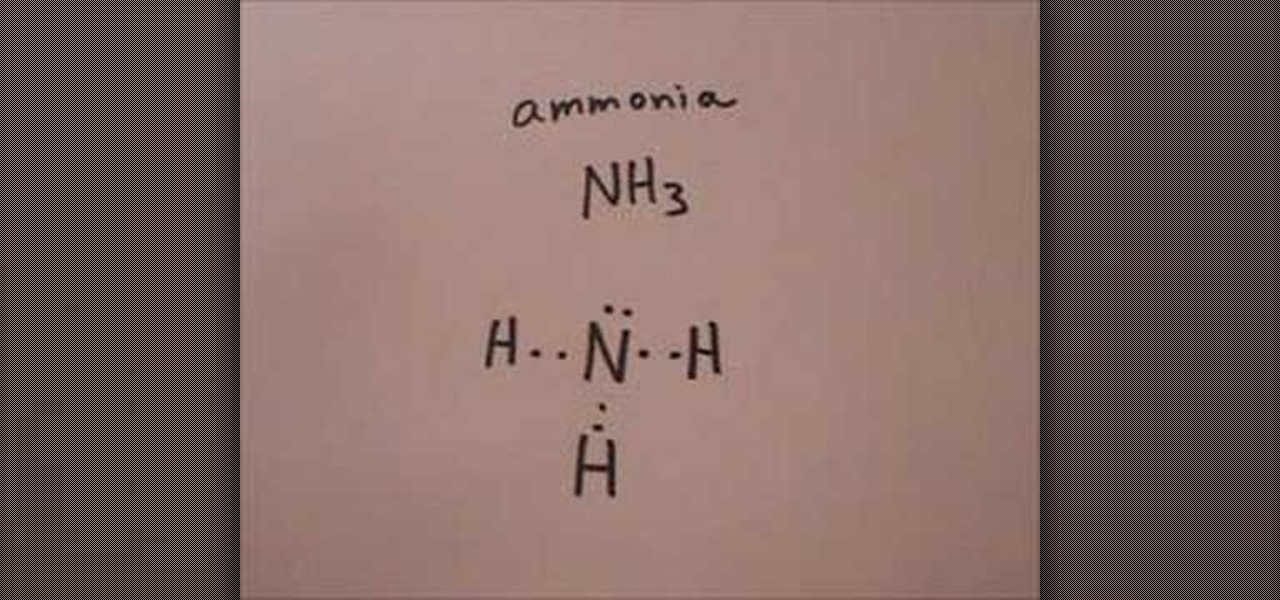
How to Draw the Lewis structure for ammonia « Science Experiments
Ammonia Lewis Dot Structure Draw Electron Dot Diagram Of Ammonia Hot

Молекула аммиака рисунок 83 фото
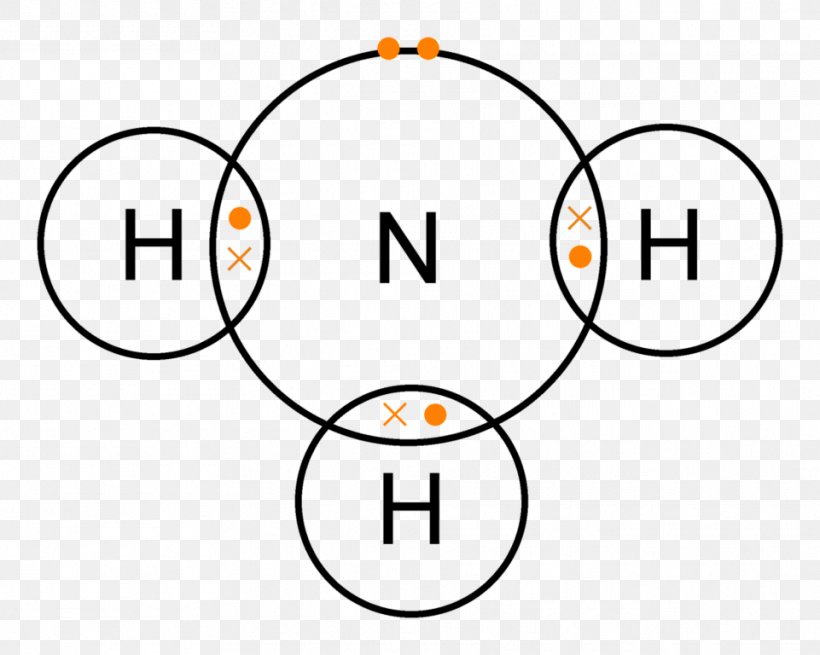
Lewis Structure Ammonia Covalent Bond Lone Pair Chemical Bond, PNG
Lewis Structure Ammonia Molecular Geometry Molecule Ammonium PNG
A Lewis Structure Is A Way To Show How Atoms Share Electrons When They Form A Molecule.
Lewis Structures Show All Of The Valence Electrons In An Atom Or Molecule.
Write Lewis Symbols For Neutral Atoms And Ions.
There Are 8 Valence Electrons Available For The Lewis Structure For Nh 3.
Related Post: