Draw The Lewis Structure For So3
Draw The Lewis Structure For So3 - Location of sulfur and oxygen on the periodic table | image: Now have a look of lewis structure again; Web 76k views 3 years ago. When we draw it, firstly we get the three structures at the top. Give the molecular geometry select) d. Web added jun 9, 2014 by webtester in chemistry. Find more chemistry widgets in wolfram|alpha. Drawing the lewis structure for so3. 6 + (3 x 6) = 24. Write lewis structures for the following: Remember, sulfur is in period 3 and can hold more than 8 valence electrons. Does it have polar covalent bonds? The lewis structure for so 3 is requires you to place more than 8 valence electrons on sulfur (s). Here, the given molecule is so3 (sulfur trioxide). You'll get a detailed solution from a subject matter expert that helps you. The valence electrons are the electrons in the. Web 639k views 9 years ago lewis structures. Web so 3 is the primary contributer to acid rain in the atomsphere. A lewis structure is a way to show how atoms share electrons when they form a molecule. In order to find the total valence electrons in so3 (sulfur trioxide) molecule, first. Does it have polar covalent bonds? Lewis structures show all of the valence electrons in an atom or molecule. Determine the total number of valence electrons in the so3 molecule. For the so3 structure use the periodic table to find the total number. The valence electrons are the electrons in the. Now have a look of lewis structure again; Does it have polar covalent bonds? 6 steps to draw the lewis structure of so3. In order to draw the lewis structure of so3, first of all you have to find the total number of valence electrons present in the so3 molecule. In this case, sulfur (s) has 6 valence electrons, and. It discusses the molecular geometry, bond angle, hybridization, and formal. Write lewis structures for the following: Get the free lewis structure finder widget for your website, blog, wordpress, blogger, or igoogle. Determine the total number of valence electrons in the so3 molecule. Is the molecule polar or nonpolar? Sulfur dioxide lewis structure is drawn step by step using vespr theory rules. Find the total valence electrons in so3 molecule. Get the free lewis structure finder widget for your website, blog, wordpress, blogger, or igoogle. When we draw it, firstly we get the three structures at the top. You might think you've got the correct lewis structure for so. Web in so3 lewis structure, there are three double bonds between sulfur atom and oxygen atoms. There are three resonance structures so3 (sulfur trioxide). Draw the lewis structure for the sulfur trioxide (so_3) molecule. The initial step in sketching the so 3 lewis structure is to determine the total number of valence electrons. Location of sulfur and oxygen on the. (please note, none of the solutions are using the expanded octet rule or formal charges) h 2. You might think you've got the correct lewis structure for so 3 at first. There are three resonance structures so3 (sulfur trioxide). 43k views 1 year ago. Draw the lewis structure for the sulfur trioxide (so_3) molecule. 43k views 1 year ago. Draw the lewis structure for the sulfur trioxide (so_3) molecule. You might think you've got the correct lewis structure for so 3 at first. Location of sulfur and oxygen on the periodic table | image: When you draw the lewis structure, you first get the three structures at the top. Web steps of drawing so3 lewis structure. Now have a look of lewis structure again; This problem has been solved! Drawing the lewis structure for so3. Remember, sulfur is in period 3 and can hold more than 8 valence electrons. Be sure to check the formal charges for the lewis structure for so 3. Calculate the total number of valence electrons. Web 639k views 9 years ago lewis structures. Note that so3 is a. Sulfur dioxide lewis structure is drawn step by step using vespr theory rules. (please note, none of the solutions are using the expanded octet rule or formal charges) h 2. These atoms are connected by double bonds, and each oxygen atom has two lone pairs of electrons. Remember, sulfur is in period 3 and can hold more than 8 valence electrons. Web how to draw lewis structures. You might think you've got the correct lewis structure for so 3 at first. Draw the lewis structure for the sulfur trioxide (so_3) molecule. This problem has been solved! Web the lewis structure of sulfur trioxide (so3) molecule is drawn by: There are seven resonance structures for so3. Does it have polar covalent bonds? Web so 3 is the primary contributer to acid rain in the atomsphere.
Draw The Lewis Dot Structure For So3 2 slidesharedocs

draw the lewis structure for so3 and answer the following questions

SO3 Lewis Structure How to Draw the Lewis Structure for SO3 (Sulfur

Draw the Lewis dot structure for SO3 Brainly.in

SO3 Lewis StructureLewis structure of SO3 (Sulfur trioxide) YouTube

SO3 Lewis Structure, Molecular Geometry, and Hybridization

SO3 Lewis Structure, Molecular Geometry, and Hybridization
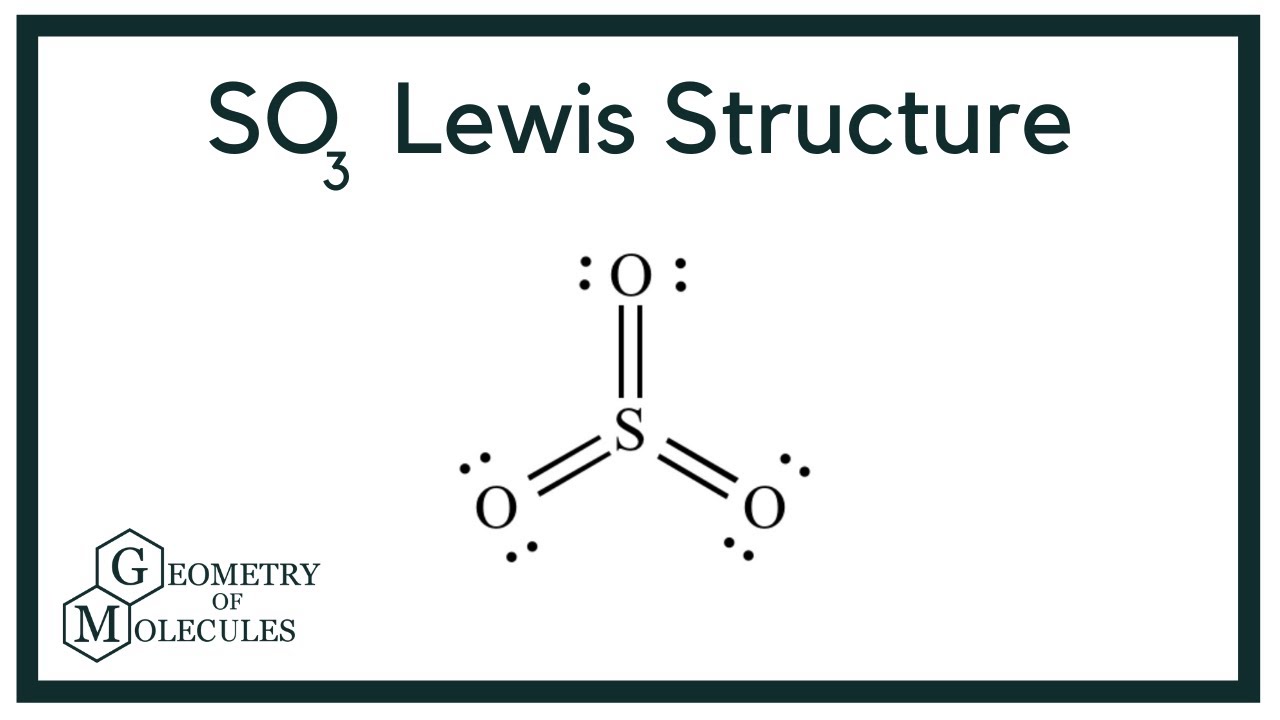
SO3 Lewis Structure (Sulfur Trioxide) YouTube
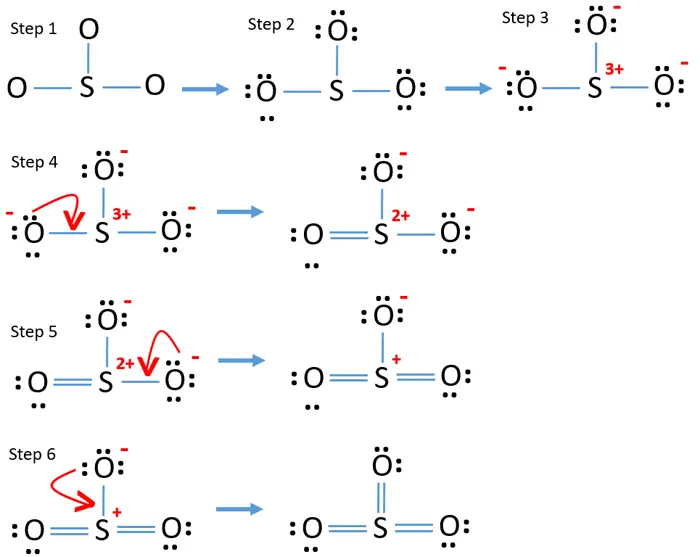
steps of drawing SO3 lewis structure VSEPR method

Draw the Lewis dot structure for SO3 Brainly.in
Web 76K Views 3 Years Ago.
The Lewis Structure For So 3 Is Requires You To Place More Than 8 Valence Electrons On Sulfur (S).
There Are 32 Valence Electrons Available For The Lewis Structure For So 3.
A Lewis Structure Is A Way To Show How Atoms Share Electrons When They Form A Molecule.
Related Post: