Draw The Lewis Structure For C2H2
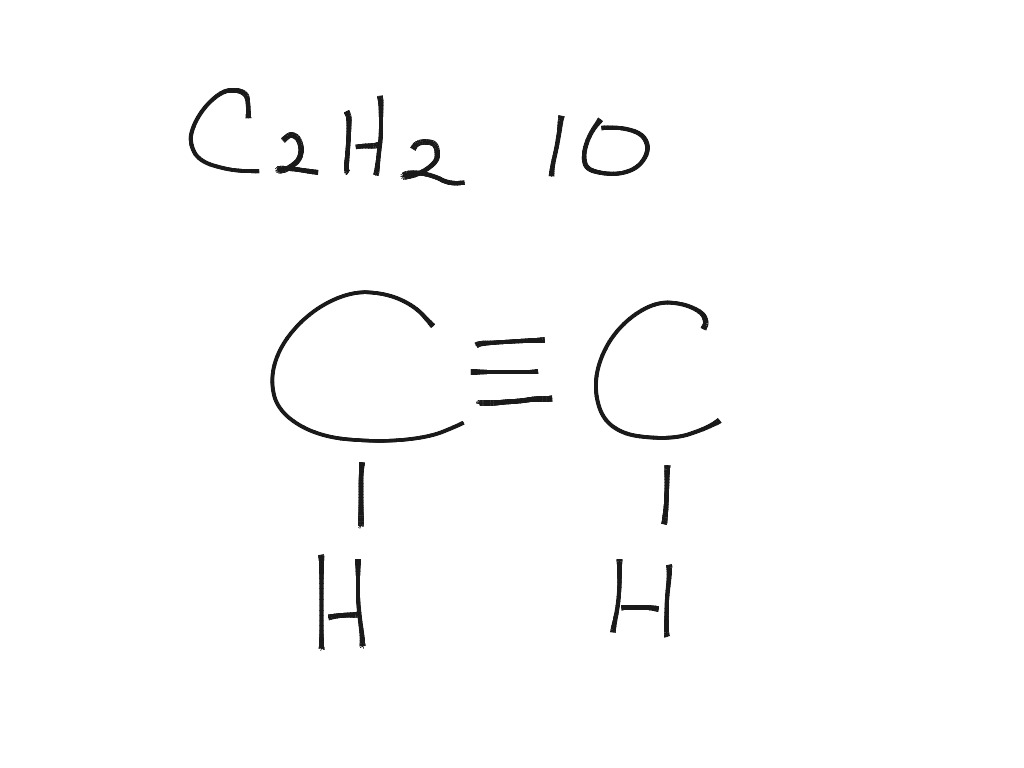
Draw The Lewis Structure For C2H2 - Remember that hydrogen (h) atoms always go on the outside of a lewis structure. Web draw lewis structures depicting the bonding in simple molecules. Steps of drawing the lewis structure of c 2 h 2. The following steps can be followed to draw the c2h2 lewis structure: Web here are the steps to draw the c2h2 lewis structure in an active voice and concise manner: Steps of drawing c2h2 lewis structure. Web to draw a lewis structure of any molecule and understand the bond formation in any molecule, it is essential to know the total number of valence electrons. #1 first draw a rough sketch. 176k views 12 years ago every video. Add together the valence electrons from each atom. Is it polar or nonpolar ? There are several steps to draw a. Determine the total number of valence electrons in the molecule by adding the valence electrons of each atom. I quickly take you through how to draw the lewis structure of chch (acetylene or ethyne). Determine the total number of valence electrons in the molecule or ion. To study this, first, it is crucial to know the electronic configuration of the participating elements. Web you can see the lewis structure of c 2 h 2 in above figure and you can see it is a simple structure. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. The valence electrons are. Include all lone pairs of electrons.draw the lewis structure for c2h4 (whose skeletal structure is h2cch2 ). Calculate the total number of valence electrons in the molecule. The following procedure can be used to construct lewis electron structures for simple molecules. Determine the intermolecular forces for each structure. What is the hybridization of the central atom in c2h2 d. Include all lone pairs of electrons.draw the lewis structure for c2h4 (whose skeletal structure is h2cch2 ). There are several steps to draw a. I quickly take you through how to draw the lewis structure of chch (acetylene or ethyne). #3 calculate and mark formal charges on the atoms, if required. What is the hybridization of the central atom in. Web lewis structure for c2h2 (ethyne) commonly tested lewis structures. A lewis structure is a way to show how atoms share electrons when they form a molecule. Once we know the valence electrons we will start making the lewis. We experienced a large temperature swing during a softball. Draw a lewis structure for ethylene, c2h2. The valence electrons are the electrons in the. Draw the lewis structures of c2h6, c2h4, and c2h2.draw the molecules by placing atoms on the grid and connecting them with bonds. This problem has been solved! #2 mark lone pairs on the atoms. Web to understand the lewis structure of c2h2,we will first calculate the total number of valence electrons for. What is the electron group geometry of c2h2? Draw the lewis structure for c2h2 (whose skeletal structure is hcch ). Determine the total number of valence electrons in the molecule or ion. The valence electrons are the electrons in the. We draw lewis structures to predict: Take a pen and paper with you and try to draw this lewis structure along with me. Web to understand the lewis structure of c2h2,we will first calculate the total number of valence electrons for this molecule. Determine the total number of valence electrons in the molecule or ion. The valence electrons are the electrons in the. What is the. Web how do you draw the lewis structure for c2h2?description:if you want to draw the lewis structure of c2h2 ,at first ,you have to count the valence electrons. Remember that hydrogen (h) atoms always go on the outside of a lewis structure. We experienced a large temperature swing during a softball. In order to find the total valence electrons in. The valence electrons are the electrons in the. Valence electrons of carbon atom +. #3 calculate and mark formal charges on the atoms, if required. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Web lewis structure of c2h2 (or acetylene or ethyne) contains one triple bond between the two carbon (c) atoms. Find the total valence electrons for the c2h2 molecule. Draw the lewis structure for c2h2 (whose skeletal structure is hcch ). Web lewis structure of c2h2 (or acetylene or ethyne) contains one triple bond between the two carbon (c) atoms and two single bonds between carbon (c) & hydrogen (h) atoms. In all cases, these bonds involve the sharing or transfer of. Web lewis structure for c2h2 (ethyne) commonly tested lewis structures. #1 first draw a rough sketch. Draw the lewis structures of c2h6, c2h4, and c2h2.draw the molecules by placing atoms on the grid and connecting them with bonds. Is it polar or nonpolar ? Determine the total number of valence electrons in the molecule by adding the valence electrons of each atom. Once we know the valence electrons we will start making the lewis. The valence electrons are the electrons in the. There are several steps to draw a. Lewis structure of acetylene (c2h2) lewis structure is the pictorial representation showing how the valence electrons are participating in bond formation. (1 pts) h h h h h 8. A lewis structure is a way to show how atoms share electrons when they form a molecule. The hybridization of c is o o o o o for the system nh2oh + ch3nh3+ ch3nh2 + nh3oh+ the position of the equilibrium lies to the left.
C2H2 Lewis Structure (Ethyne or Acetylene) Math, Lewis, Molecules

C2H2 Lewis Structure, Molecular Geometry, Hybridization, Polarity, and
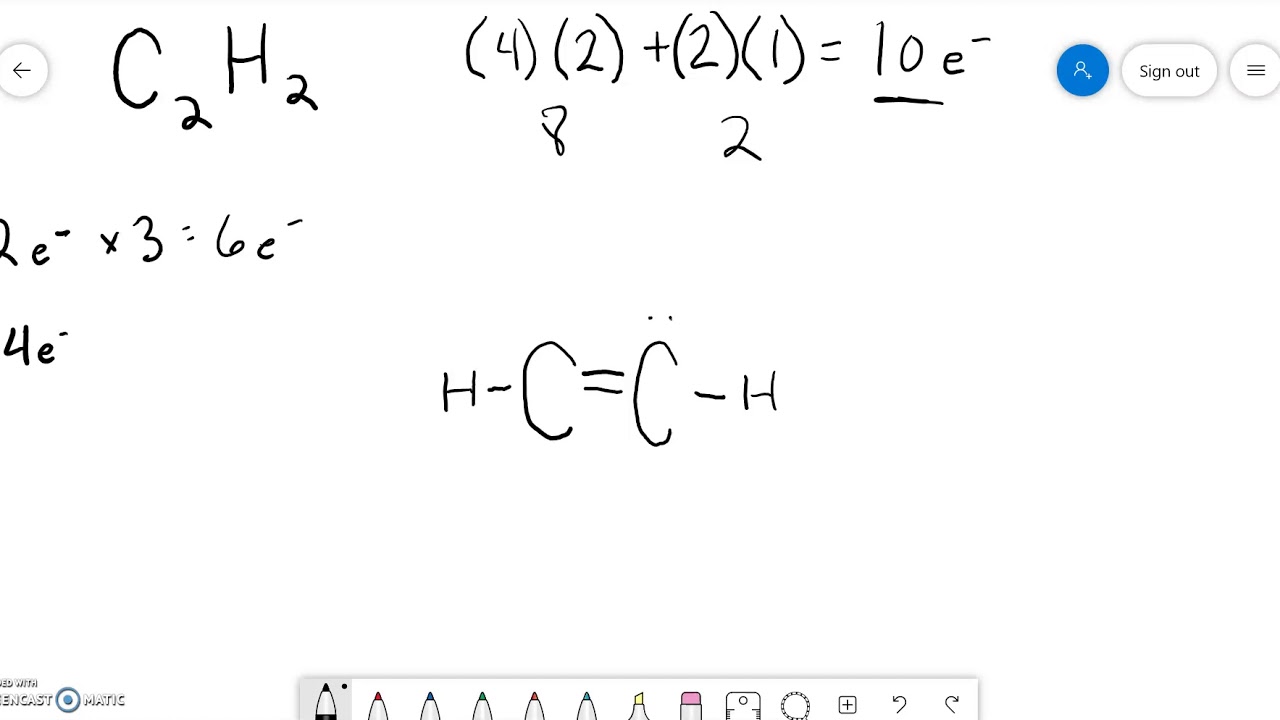
Lewis Structure C2H2 YouTube

How do you draw the Lewis structure for C2H2? Ethyne or Acetylene

How to Draw the Lewis Dot Structure for C2H2 Acetylene (Ethyne) YouTube
C2h2 Lewis Structure

C2H2 Molecular Geometry / Shape and Bond Angles (see description for

41 lewis dot diagram for c2h2 Diagram For You

C2H2 Lewis structure ,Valence Electrons, Formal Charge

C2H2 Lewis Structure, Molecular Geometry, Hybridization, Polarity, and
#3 Calculate And Mark Formal Charges On The Atoms, If Required.
Let’s Draw And Understand This Lewis Dot Structure Step By Step.
Identify The Central Atom In The Molecule.
What Is The Hybridization Of The Central Atom In C2H2 D.
Related Post: