Draw The Lewis Structure For Brf3
Draw The Lewis Structure For Brf3 - In the brf 3 lewis structure bromine (br) is the least electronegative atom and goes in the center of the lewis structure. Drawing the lewis structure for brf3. Web here is what is needed: It is helpful if you: The brf3 lewis structure consists of one central atom, bromine (br), and three outer atoms, fluorine (f), at a bond angle of. 2.1k views 11 months ago. Web to draw lewis structures for molecules and polyatomic ions with one central atom. Web use these steps to correctly draw the brf 3 lewis structure: I beleive it should look like this: Draw the lewis dot structure for the molecule brf3. #3 calculate and mark formal charges. 35k views 11 years ago chemistry lewis dot structures. Brf3 does not follow the octet rule. Write the correct skeletal structure for the molecule. The name of the lewis structure for brf3 is bromine trifluoride. A video explanation of how to draw the lewis dot structure for. Write the correct skeletal structure for the molecule. Interactive 3d chemistry animations of reaction mechanisms and 3d models of chemical structures for students studying university. The name of the lewis structure for brf3 is bromine trifluoride. Web average rating 3.8 / 5. In the brf 3 lewis structure bromine (br) is the least electronegative atom and goes in the center of the lewis structure. Write the correct skeletal structure for the molecule. Web to draw lewis structures for molecules and polyatomic ions with one central atom. Try to draw the brf 3 lewis. Web here is what is needed: Web lewis structure, brf3 molecular and electron geometry based on the vsepr theory, the steric number, hybridization and expected bond angles. Web use these steps to correctly draw the brf 3 lewis structure: Steps of drawing brf3 lewis structure. Both br and f have seven. Web draw the lewis structure for brf3 and choose all the statements below that are. * hydrogen atoms are always terminal (only. Draw the lewis dot structure for the molecule brf3. Web to draw lewis structures for molecules and polyatomic ions with one central atom. For the brf structure use the. The brf3 lewis structure consists of one central atom, bromine (br), and three outer atoms, fluorine (f), at a bond angle of. A lewis structure is a way to show how. #3 calculate and mark formal charges. 23k views 10 years ago. Web average rating 3.8 / 5. Web the lewis structure of brf3 can be represented as follows: Drawing the lewis structure for brf3. The brf3 lewis structure consists of one central atom, bromine (br), and three outer atoms, fluorine (f), at a bond angle of. Write the correct skeletal structure for the molecule. Web lewis structure, brf3 molecular and electron geometry based on the vsepr theory, the steric number, hybridization and expected bond angles. 2.1k views 11. * hydrogen atoms are always terminal (only. The brf3 lewis structure consists of one central atom, bromine (br), and three outer atoms, fluorine (f), at a bond angle of. Find the total valence electrons in brf3 molecule. For the brf structure use the. Both br and f have seven. 23k views 10 years ago. Find the total valence electrons in brf3 molecule. 35k views 11 years ago chemistry lewis dot structures. Drawing the lewis structure for brf3. Web drawing the lewis structure for brf 3. Write the correct skeletal structure for the molecule. In the brf 3 lewis structure bromine (br) is the least electronegative atom and goes in the center of the lewis structure. Interactive 3d chemistry animations of reaction mechanisms and 3d models of chemical structures for students studying university. Web the lewis structure of brf3 can be represented as follows: In order. Web average rating 3.8 / 5. The name of the lewis structure for brf3 is bromine trifluoride. A lewis structure is a way to show how. Web drawing the lewis structure for brf 3. A video explanation of how to draw the lewis dot structure for. Draw the lewis dot structure for the molecule brf3. #3 calculate and mark formal charges. #2 mark lone pairs on the atoms. 35k views 11 years ago chemistry lewis dot structures. Web to draw lewis structures for molecules and polyatomic ions with one central atom. Web use these steps to correctly draw the brf 3 lewis structure: In order to find the total valence electrons in a brf3. The brf3 lewis structure consists of one central atom, bromine (br), and three outer atoms, fluorine (f), at a bond angle of. Web draw the lewis structure for brf3 and choose all the statements below that are true for this molecule. * hydrogen atoms are always terminal (only. In the brf 3 lewis structure bromine (br) is the least electronegative atom and goes in the center of the lewis structure.
Formal charge on bromine atom of BrF3 molecule = (7 4(6/2)) =0

draw the lewis structure for brf3 in the window below and then decide

Leave a Comment Cancel Reply
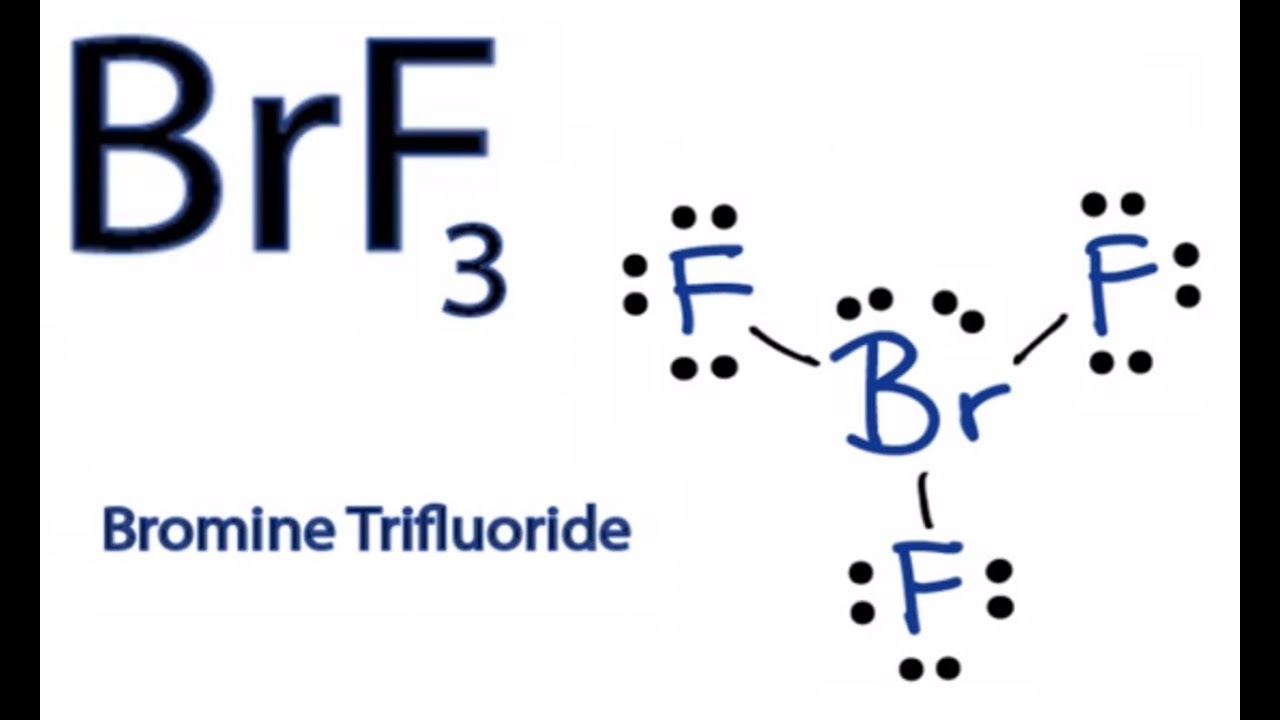
How to Draw the Lewis Dot Structure for BrF3 Boron trifluoride YouTube

Structure de Brf3 Lewis, caractéristiques 13 faits à connaître

Estructura de Brf3 Lewis, características 13 datos que debe conocer

Brf3 Molecule
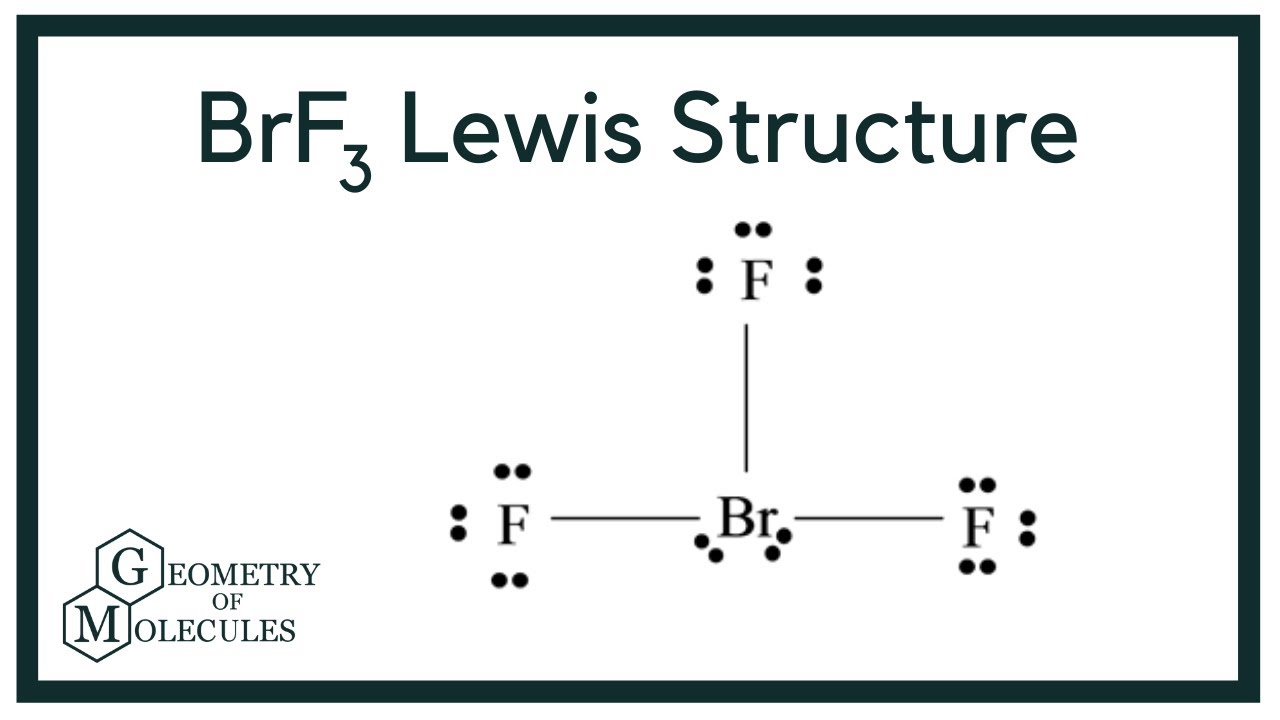
BrF3 Lewis Structure (Bromine Trifluoride) YouTube

BrF3 Lewis Structure, Molecular Geometry, Hybridization, and MO Diagram

BrF3 Lewis Structure, Molecular Geometry, Hybridization, and MO Diagram
#1 First Draw A Rough Sketch.
Find The Total Valence Electrons In Brf3 Molecule.
Try To Draw The Brf 3 Lewis.
Write The Correct Skeletal Structure For The Molecule.
Related Post: