Draw The Lewis Structure For Bf3
Draw The Lewis Structure For Bf3 - Calculate the total number of valence electrons. In this video, we are going to draw the lewis structure for. The number of lone pairs = the number of single bonds = the number of double bonds = 2. Boron atom is the center atom. Find the total valence electrons in bf3 molecule. Web 317k views 10 years ago. Watch the video and see if you missed any steps or information. O 180 o 109.5 o $109.5 o 120. Boron (b) doesn't need 8 valence electrons to have an octet (boron often only needs 6). In this tutorial, we will learn how to draw the lewis structure of bf 3 with all theories. Web bf3 draw the lewis structure for bf3 in the box at the right, including lone pairs. As discussed, here there are 24 electrons. The central boron atom a. 109.5∘ 120∘ <109.5∘ 180∘ what is the molecular shape of bf3 ? Steps of drawing bf3 lewis structure. For the central boron atom: For bf3, the total count of. Web it is isoelectronic with carbonate anion. What is the f−b−f bond angle? #1 first draw a rough sketch. Polar.the molecule bf3 is nonpolar. Watch the video and see if you missed any steps or information. Web check me out: Linear bent tetrahedral trigonal planar trigonal pyramidal the b−f bond in bf3 is nonpolar. Try structures similar to o 3 for more practice. If you're not sure you have the best lewis structure for bf 3 you can calculate the formal charges. The bf 3 lewis structure is similar to bcl 3 and bbr 3 since cl and br are in group 7 and have 7 valence electrons. #1 first draw a rough sketch. This shape makes an equilateral triangle with each side. A prerequisite for accurately portraying the bf3 lewis structure is determining the cumulative valence electrons. First, determine the total number of valence electrons. #1 first draw a rough sketch. This problem has been solved! Web to draw a lewis structure, first of all, add electrons and draw the connectivities. € what is the the shape (molecular geometry) of bf3? 109.5∘ 120∘ <109.5∘ 180∘ what is the molecular shape of bf3 ? Try to draw the bf 3 lewis structure before watching the video. Web it is isoelectronic with carbonate anion. Try structures similar to o 3 for more practice. Boron atom is the center atom. However, it is hard to imagine that one rule could be followed by all molecules. #1 first draw a rough sketch. What is the f−b−f bond angle? Here, the given molecule is bf3. But, as we know, there are no extra electrons. Boron atom is the center atom. 109.5∘ 120∘ <109.5∘ 180∘ what is the molecular shape of bf3 ? In order to find the total valence electrons in bf3 molecule, first of all you should know the valence electrons present in boron atom as well as fluorine atom. Steps of drawing bf3. This problem has been solved! 375 views 3 years ago covalent bonding. Steps of drawing bf3 lewis structure. In order to find the total valence electrons in bf3 molecule, first of all you should know the valence electrons present in boron atom as well as fluorine atom. If you're not sure you have the best lewis structure for bf 3. Try structures similar to o 3 for more practice. Web lewis structure of boron trifluoride (bf 3) is shown below and you can see each fluorine atom has made a single bond with boron atom. Web how to draw lewis structure for bf3 boron trifluoride?lewis structure: For bf3, the total count of. Calculating the total valence electrons. Boron (b) doesn't need 8 valence electrons to have an octet (boron often only needs 6). Valence electrons in boron (b): Polar.the molecule bf3 is nonpolar. • do not include overall ion charges or formal charges in your drawing. This shape makes an equilateral triangle with each side making a. In this tutorial, we will learn how to draw the lewis structure of bf 3 with all theories. € what is the the shape (molecular geometry) of bf3? Web it is helpful if you: Steps of drawing bf3 lewis structure. Web use these steps to correctly draw the bf 3 lewis structure: Try to draw the bf 3 lewis structure before watching the video. Try structures similar to o 3 for more practice. Watch the video and see if you missed any steps or information. A lewis structure is a way to show how atoms share electrons when they form a molecule. Lewis structures show all of the valence electrons in an atom or molecule. Web how to draw the lewis dot structure for bf3 :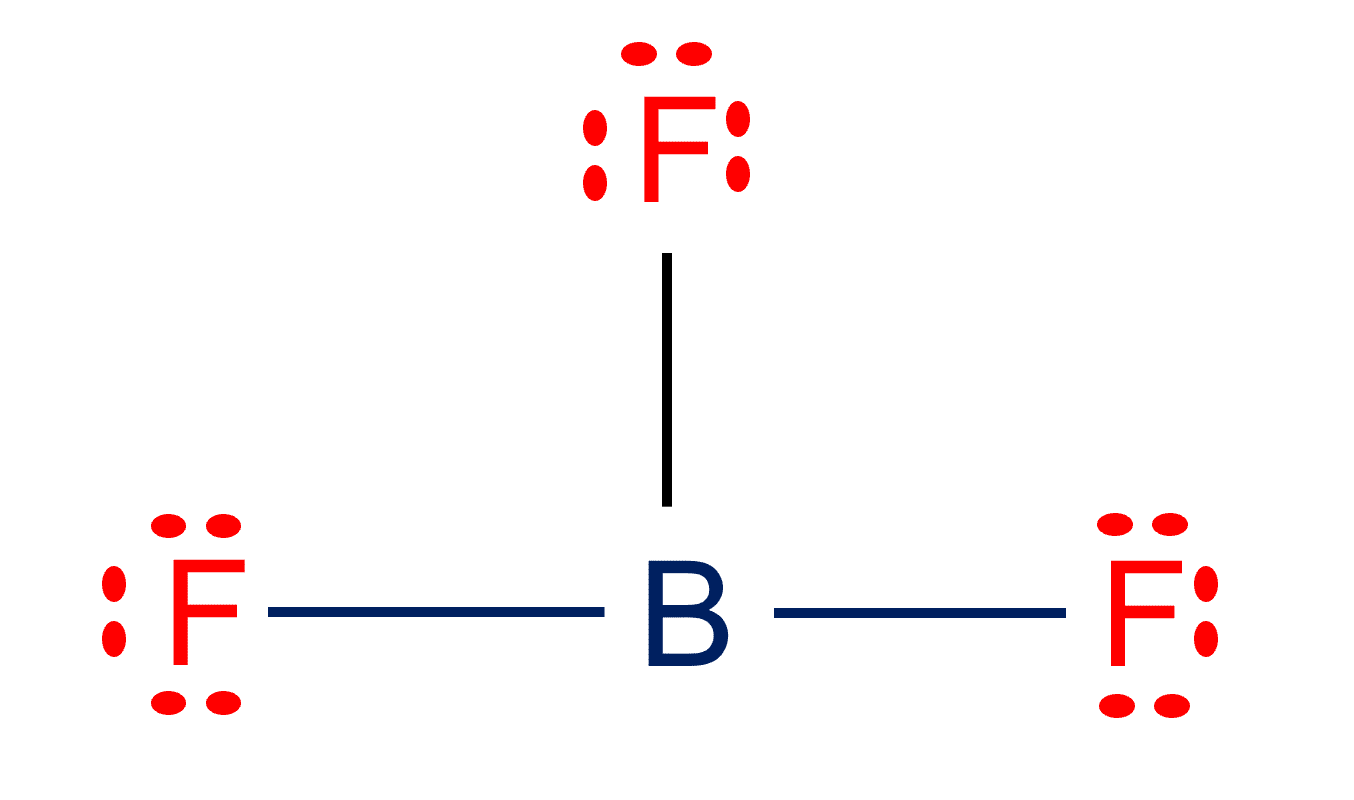
BF3 Lewis structure Molecular geometry, Hybridization, and Polarity

How to draw BF3 Lewis Structure? 5

BF3 lewis structure and Molecular Geometry YouTube

BF3 Molecular Geometry Science Education and Tutorials

BF3 Lewis Structure (Boron Trifluoride) YouTube

Bf3 Lewis Structure Lone Pairs Draw Easy

How to Draw the Lewis Dot Structure for BF3 Boron trifluoride YouTube

BF3 Lewis Structure How to Draw the Lewis Structure for BF3 YouTube

Bf3 Lewis Structure Molecular Geometry And Hybridization itechguide
Molecular Geometry CK12 Foundation
To Accurately Depict The Lewis Structure For Bf3 (Boron Trifluoride) Follow The Below Steps:
(Valence Electrons Are The Number Of Electrons Present In The Outermost Shell Of An Atom).
375 Views 3 Years Ago Covalent Bonding.
Web It Is Isoelectronic With Carbonate Anion.
Related Post:
