Draw The Lewis Structure For A Nitrogen Molecule
Draw The Lewis Structure For A Nitrogen Molecule - Write lewis symbols for neutral atoms and ions. The structure consists of a triple bond between the two nitrogen atoms and lone pairs on one of the nitrogen atoms. When the substance of nitrogen melts, what interaction (s) are overcome? Transcribed image text:a 'h nmr spectrum is shown for a molecule with the molecular formula of c5h10o2. Thus far in this chapter, we have discussed the various types of bonds that form between atoms and/or ions. Web in the lewis structure of the n2 molecule, there is a formation of a triple covalent bond represented by three lines between two atoms of nitrogen. Calculate the number of valence electrons: Web so, nitrogen is in group 5a, and therefore, it has 5 valence electrons, thus n2 has 10 valence electrons. Draw the lewis structure for a nitrogen (n2) molecule. Thus far in this chapter, we have discussed the various types of bonds that form between atoms and/or ions. Lewis structure of n2 molecule contains a triple bond and each nitrogen atom has one lone pair. Write lewis symbols for neutral atoms and ions. How to draw a lewis structure. Thus far in this chapter, we have discussed the various types of bonds that form between atoms and/or ions. Draw lewis structures depicting the bonding in simple molecules. The structure consists of a triple bond between the two nitrogen atoms and lone pairs on one of the nitrogen atoms. There are 10 valence electrons available for the lewis structure for n 2. In this case, we can condense the last few steps, since not all of them apply. Draw lewis structures depicting the bonding in simple molecules. Web. This problem has been solved! Draw the structure that best fits this data. Web so, nitrogen is in group 5a, and therefore, it has 5 valence electrons, thus n2 has 10 valence electrons. 8 + (2 × 7) = 22 xef 6: View the full answer step 2. 58k views 1 year ago. Web draw lewis structures depicting the bonding in simple molecules. By the end of this section, you will be able to: 8 + (6 × 7) = 50. 8 + (2 × 7) = 22 xef 6: By the end of this section, you will be able to: A lewis structure is a way to show how atoms share electrons when they form a molecule. Draw lewis structures depicting the bonding in simple molecules. Thus far in this chapter, we have discussed the various types of bonds that form between atoms and/or ions. Web there is a. Thus far in this chapter, we have discussed the various types of bonds that form between atoms and/or ions. Web draw a skeleton structure of the molecule. Lewis dot structure for n2. The example is for the nitrate ion. Web key to this theory is the lewis structure, which is a very simplified representation of the electrons in a molecule. Draw the lewis structure for a nitrogen molecule, n2. Web in the lewis structure of the n2 molecule, there is a formation of a triple covalent bond represented by three lines between two atoms of nitrogen. In this case, there is no central atom, so we distribute the electrons around both atoms. When the substance of nitrogen melts, what interaction. Drawing the lewis structure for n2. Lewis structure of n2 molecule contains a triple bond and each nitrogen atom has one lone pair. Draw the lewis structure for a nitrogen (n2) molecule. We also use lewis symbols to indicate the formation of covalent bonds, which are shown in lewis structures, drawings that describe the bonding in molecules and polyatomic ions.. Thus far in this chapter, we have discussed the various types of bonds that form between atoms and/or ions. + + 10 8 7 5 3 2 ppm drag to pan close undo reset drag to pan. Transcribed image text:a 'h nmr spectrum is shown for a molecule with the molecular formula of c5h10o2. Nitrogen is a diatomic molecule and. Write lewis symbols for neutral atoms and ions. 8 + (6 × 7) = 50. Web to draw the n2 lewis structure, you can follow these steps: 100% (2 ratings) share share. This problem has been solved! The example is for the nitrate ion. When the substance of nitrogen melts, what interaction (s) are overcome? There are 10 valence electrons available for the lewis structure for n 2. Web draw lewis structures depicting the bonding in simple molecules. View the full answer step 2. Transcribed image text:a 'h nmr spectrum is shown for a molecule with the molecular formula of c5h10o2. Draw lewis structures depicting the bonding in simple molecules. Draw the lewis structure for a nitrogen molecule, n2. Draw lewis structures for molecules. It also is a good example of a molecule with a triple bond. Lewis structures show all of the valence electrons in an atom or molecule. The structure consists of a triple bond between the two nitrogen atoms and lone pairs on one of the nitrogen atoms. Web key to this theory is the lewis structure, which is a very simplified representation of the electrons in a molecule and is use to show how the electrons are arranged around individual atoms in a molecule. Web draw a skeleton structure of the molecule. In the case of n2, each nitrogen atom has five valence electrons, so the total number of valence electrons is 10. By the end of this section, you will be able to:
N2 Lewis Structure How to Draw the Lewis Structure for N2 Nitrogen Gas
Lewis Electron Dot Diagram Nitrogen

Nitrogen Wikipedia

Nitrogen Gas Lewis Dot Structure For Nitrogen Gas
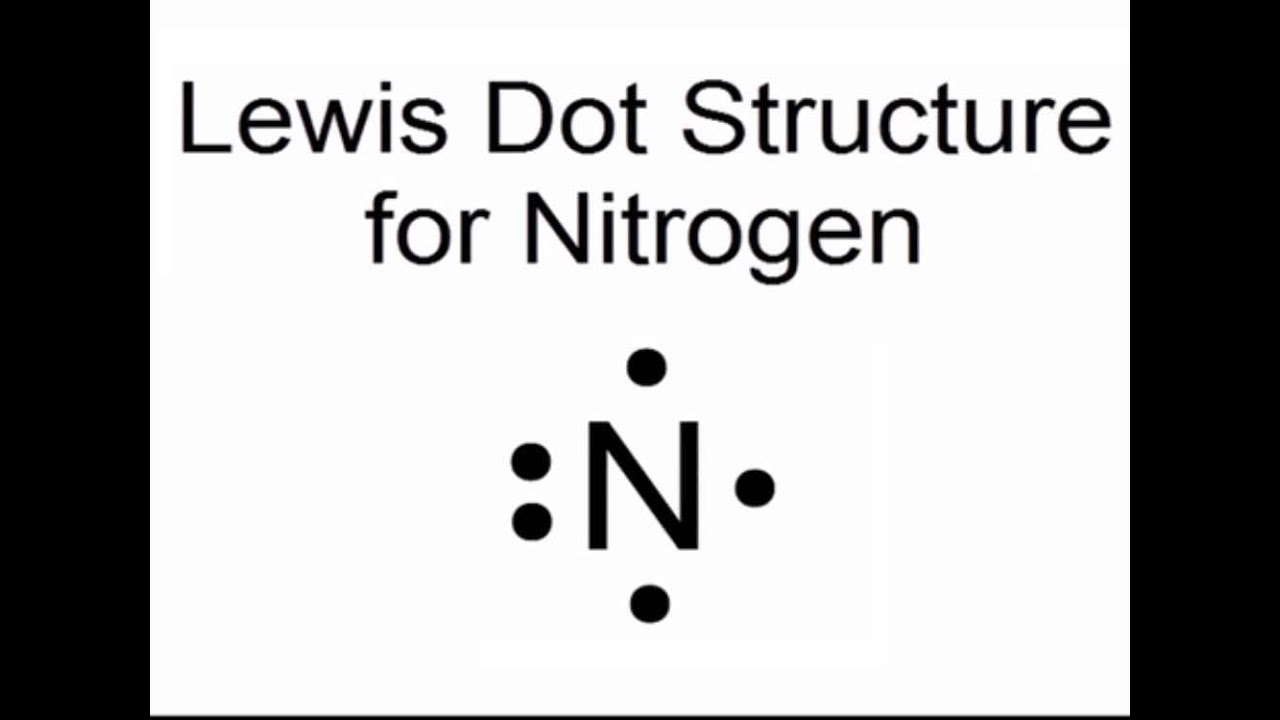
Lewis Dot Structure for Nitrogen Atom (N) YouTube

Diagram representation of the element nitrogen Vector Image
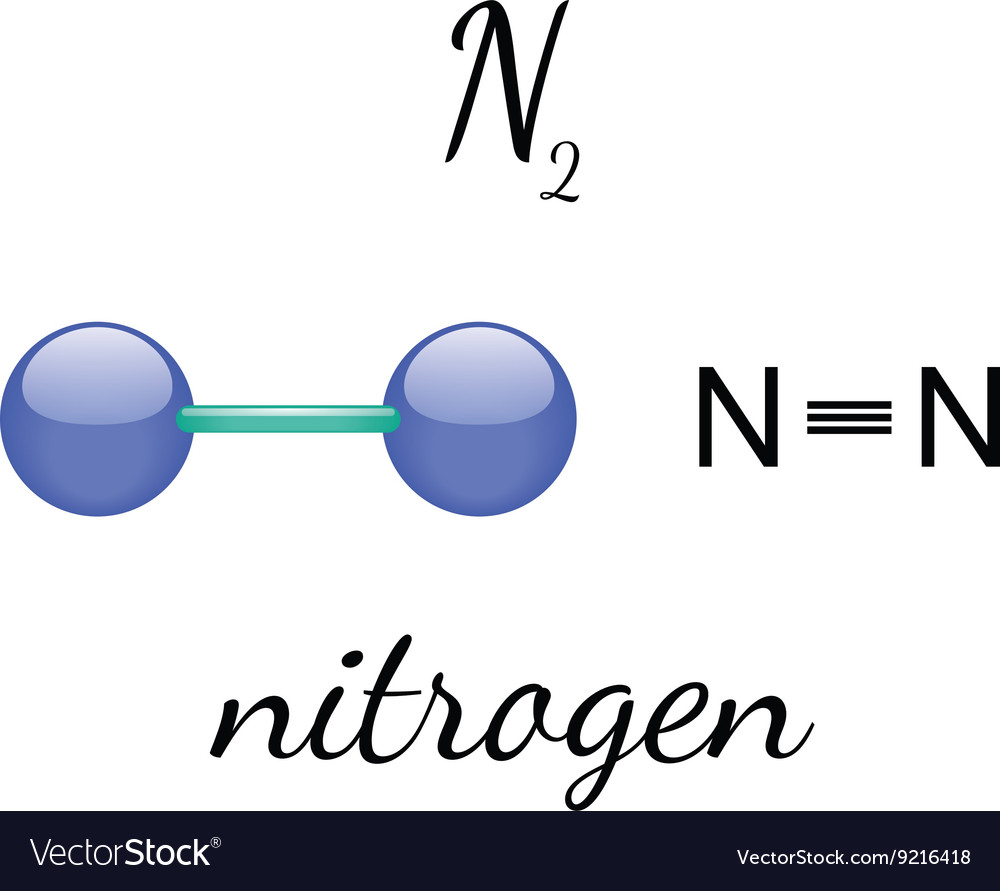
N2 nitrogen molecule Royalty Free Vector Image

Question Video Identifying the Lewis Structure of an Atom of Nitrogen
Nitrogen Facts, Symbol, Discovery, Properties, Uses
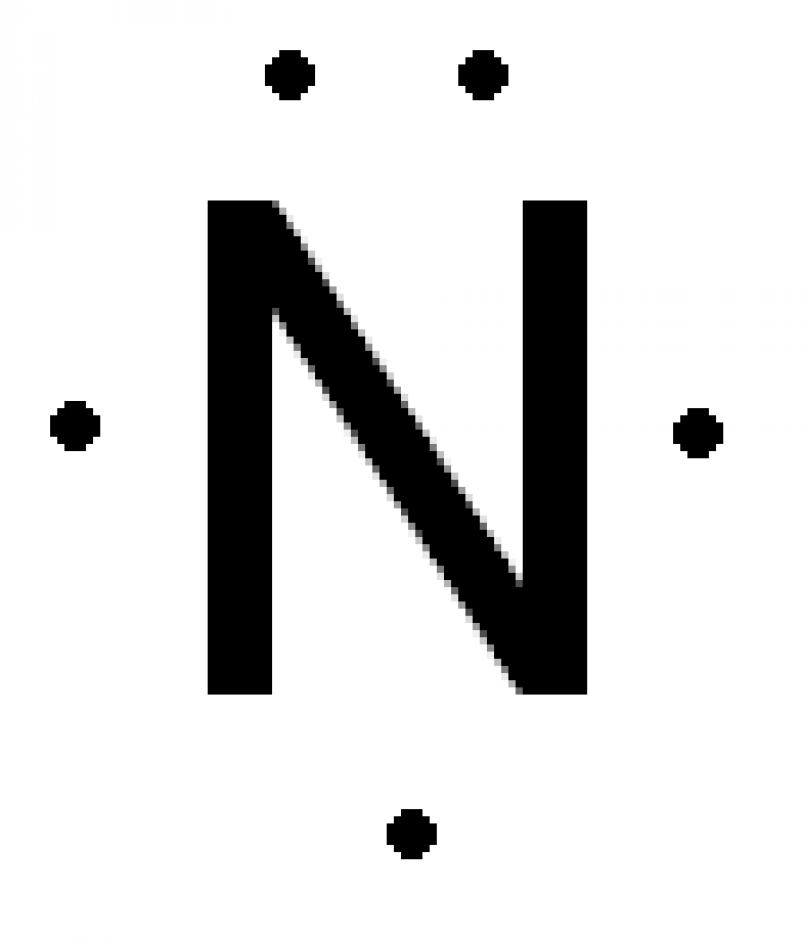
Help Me With Basic Chemistry How to Do Lewis Dot Structure (Simple)
In This Case, There Is No Central Atom, So We Distribute The Electrons Around Both Atoms.
Web To Draw The N2 Lewis Structure, You Can Follow These Steps:
Web So, Nitrogen Is In Group 5A, And Therefore, It Has 5 Valence Electrons, Thus N2 Has 10 Valence Electrons.
Using The Periodic Table To Draw Lewis Dot Structures.
Related Post:
