Draw The Electron Configuration For A Neutral Atom Of Zinc
Draw The Electron Configuration For A Neutral Atom Of Zinc - The atomic number of p is 15. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Web to check the answer, verify that the subscripts add up to the atomic number. Draw the electron configuration for a neutral atom of zinc. 1s2 2s2 2p6 3s2 3p6 3d10 4s2. Subshells are described by writing the principal quantum. What is the electron configuration of zinc. Web excited state electron configuration of zn is 1s2 2s2 2p6 3s2 3p6 4s1 3d10 4p1, which is also written as [ar] 3d10 4s1 4p1, where electrons in 4s orbit jumps into higher energy. This problem has been solved! Web today we are going to tell you the electron configuration of the zn. This can be determined by using the periodic table and filling the electron. Web using figure \(\pageindex{2}\) as your guide, write the electron configuration of a neutral phosphorus atom. We have chosen to show. The atomic number of p is 15. Web for example, [ne] represents the 1s 2 2s 2 2p 6 electron configuration of neon (z = 10),. Web excited state electron configuration of zn is 1s2 2s2 2p6 3s2 3p6 4s1 3d10 4p1, which is also written as [ar] 3d10 4s1 4p1, where electrons in 4s orbit jumps into higher energy. Therefore, its ground state electronic configuration can be written as 1s 2 2s 2 2p 6 3s 2 3p 5. Electron configuration can be done in. [ar] 3d10 4s2 is the electron configuration of zinc. What is the electron configuration of zinc. However, notice that 1s 2 2s 2 2p 6 3s 2 3p 6 is the configuration for argon, a noble gas. Therefore, its ground state electronic configuration can be written as 1s 2 2s 2 2p 6 3s 2 3p 5. Web zinc's full. Web find the atomic number of nitrogen (7) and use this electron configuration calculator to get a complete electron configuration. Web you may have an easy way to know the number of electrons in a neutral atom, but the placement of those electrons gets a little more complex. Web to check the answer, verify that the subscripts add up to. This can be determined by using the periodic table and filling the electron. Therefore, its ground state electronic configuration can be written as 1s 2 2s 2 2p 6 3s 2 3p 5. Since the atomic number of zinc is 30, the total electrons of. Web the full electron configuration of zinc is 1s2 2s22p6 3s23p63d10 4s2zinc, also written zinc,. 1s2 2s2 2p6 3s2 3p6 3d10 4s2. In this case, 2+2+6+2+6+2+10+6+2+1= 39 and z=39, so the answer is correct. Web the electron configuration of zn2+ is 1s22s22p63s23p63d10. We have chosen to show. This can be determined by using the periodic table and filling the electron. Subshells are described by writing the principal quantum. The atomic number of zinc is 30, which means that all zinc atoms have 30 protons in their nuclei. Web you may have an easy way to know the number of electrons in a neutral atom, but the placement of those electrons gets a little more complex. [ar] 3d10 4s2 is the. Web full electron configuration of zinc: You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Web to write the configuration for the zinc and the zinc ion, first we need to write the electron configuration for just zinc (zn). Web zinc's full electron configuration is: The atomic number of p is 15. Let's take a look at the. Web to check the answer, verify that the subscripts add up to the atomic number. Web find the atomic number of nitrogen (7) and use this electron configuration calculator to get a complete electron configuration. Web full electron configuration of zinc: In this case, 2+2+6+2+6+2+10+6+2+1= 39 and z=39, so the answer is correct. Let's take a look at the. Web to write the configuration for the zinc and the zinc ion, first we need to write the electron configuration for just zinc (zn). The atomic number of zinc represents the total number of electrons of zinc. In this case, 2+2+6+2+6+2+10+6+2+1= 39 and z=39, so the answer is correct. Web find the atomic number. Web the full electron configuration of zinc is 1s2 2s22p6 3s23p63d10 4s2zinc, also written zinc, is defined as the chemical element that belongs to the periodic table of elements. What is the electron configuration of zinc. Web for example, [ne] represents the 1s 2 2s 2 2p 6 electron configuration of neon (z = 10), so the electron configuration of sodium, with z = 11, which is 1s 2 2s 2 2p 6 3s 1, is. [ar] 3d10 4s2 is the electron configuration of zinc. Web find the atomic number of nitrogen (7) and use this electron configuration calculator to get a complete electron configuration. Electron configuration can be done in two ways. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Electron configurations list every subshell for an atom or ion and how many electrons are in each subshell. However, notice that 1s 2 2s 2 2p 6 3s 2 3p 6 is the configuration for argon, a noble gas. Web to check the answer, verify that the subscripts add up to the atomic number. 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10. Web the electron configuration for a neutral atom of zinc is 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10. In this case, 2+2+6+2+6+2+10+6+2+1= 39 and z=39, so the answer is correct. Let's take a look at the. Web full electron configuration of zinc: The atomic number of zinc represents the total number of electrons of zinc.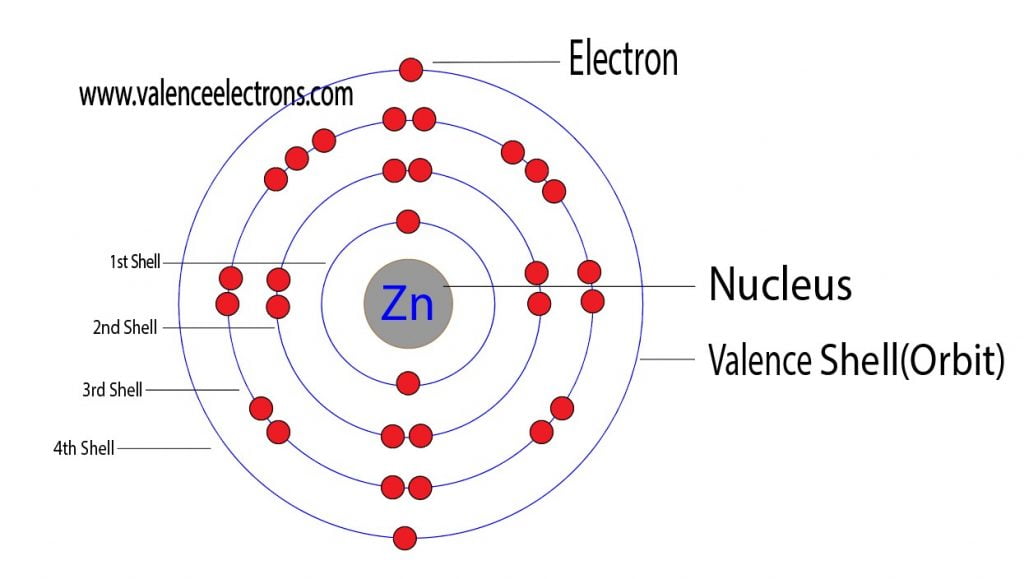
How to Find the Valence Electrons for Zinc (Zn)?

Draw The Electron Configuration For A Neutral Atom, HD Png Download
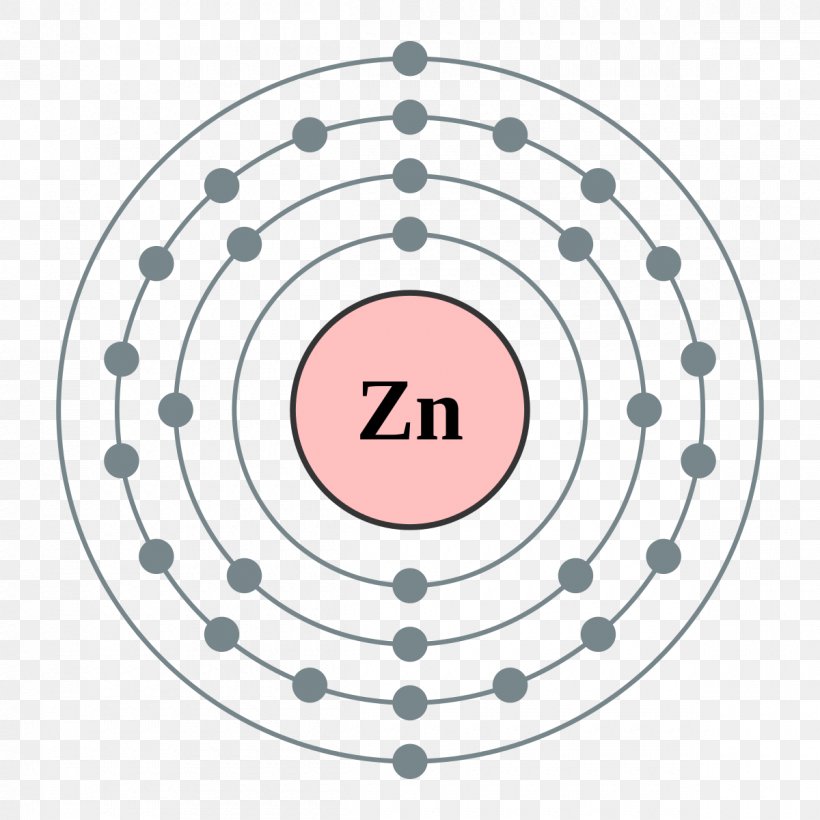
Zinc Atom Lewis Structure Bohr Model Electron Configuration, PNG
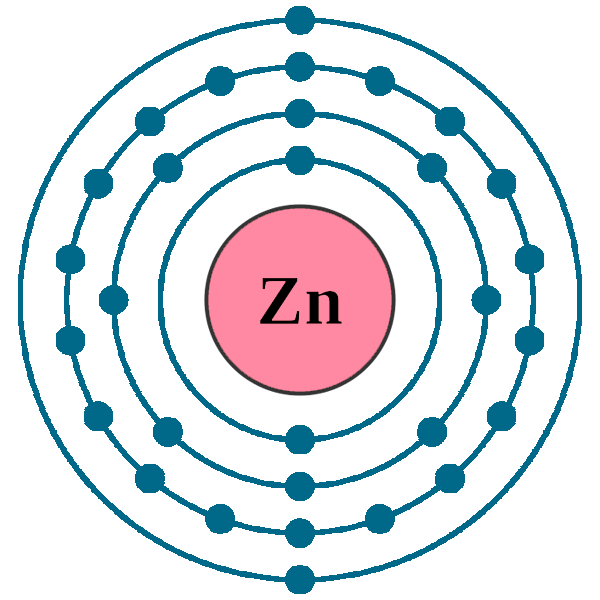
Zinc Zn (Element 30) of Periodic Table Elements FlashCards

Zinc Protons Neutrons Electrons Electron Configuration
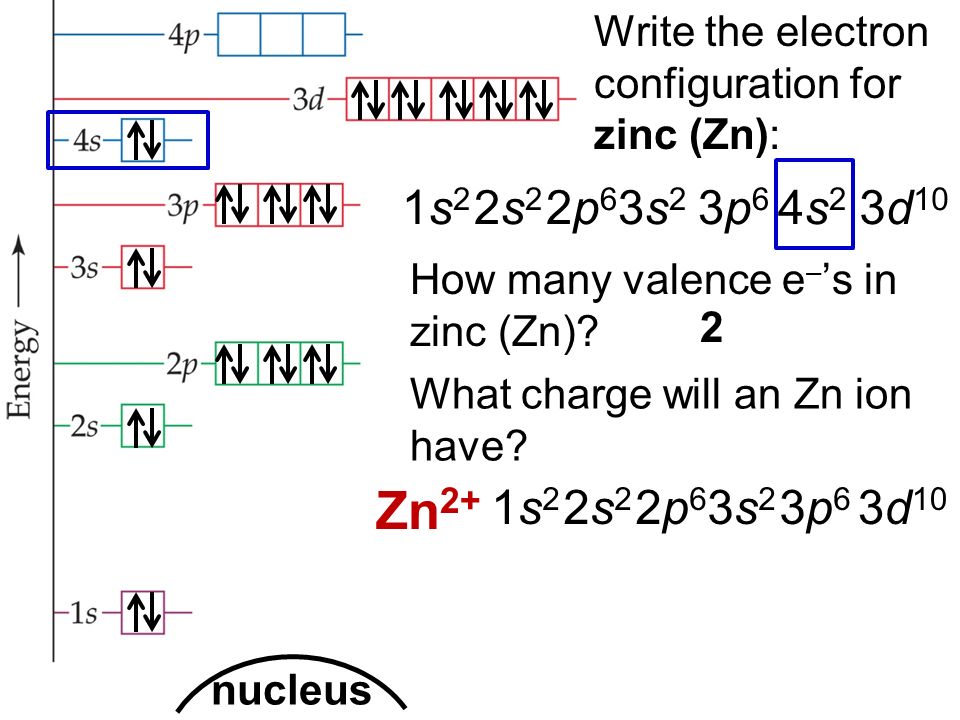
How To Find A Electron Configuration For Zinc Dynamic Periodic Table

Symbol and electron diagram for zinc Royalty Free Vector
Zinc Periodic Table Neutrons Awesome Home
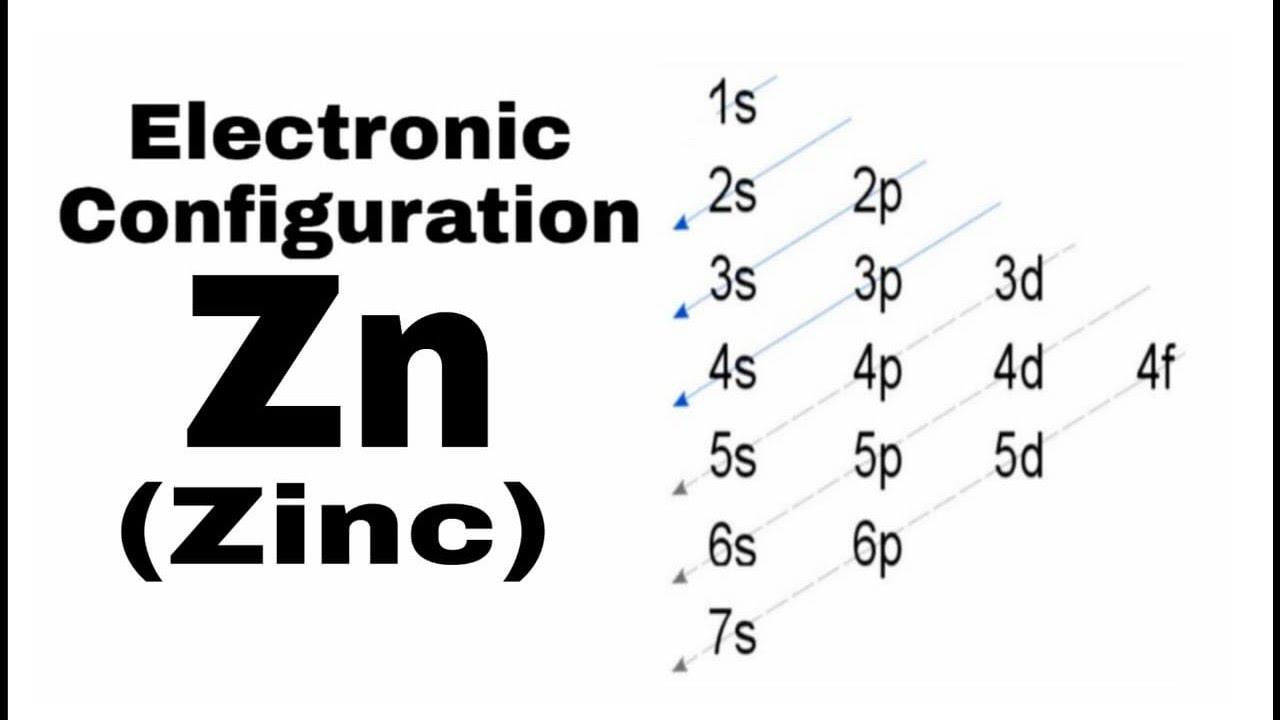
zinc electronic configuration How to Write Zinc electronic
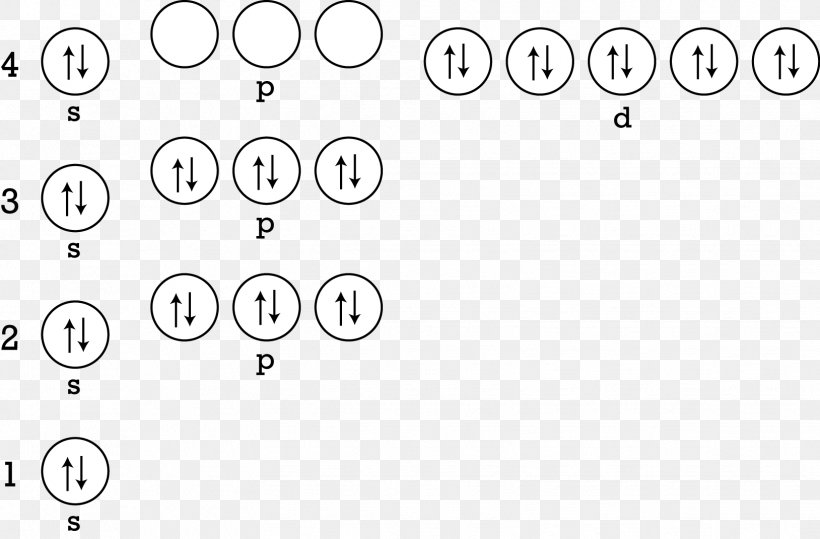
Electron Configuration Atom Zinc Energy Level, PNG, 1628x1071px
Web Today We Are Going To Tell You The Electron Configuration Of The Zn.
Web What Is The Electron Configuration Of:
This Can Be Determined By Using The Periodic Table And Filling The Electron.
We First Need To Find The Number Of Electrons For The Zn.
Related Post:
