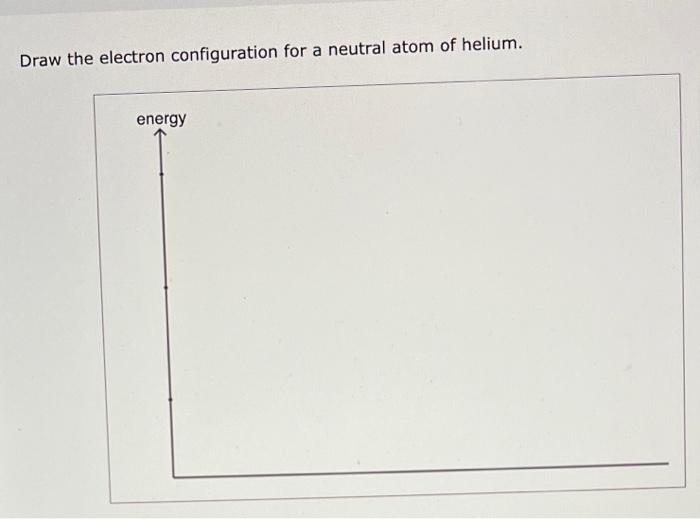
Draw The Electron Configuration For A Neutral Atom Of Helium
Draw The Electron Configuration For A Neutral Atom Of Helium - Web helium, #he#, has an atomic number equal to #2#, which means that it has #2# protons in its nucleus. To draw the electron configuration diagram for helium, we can represent the first energy level as a circle and place two dots inside it. Energy 1 l х 5 ? Both electrons fit into the 1 s subshell because s subshells can hold up to 2 electrons; Draw the electron configuration for a neutral atom of helium. Make certain that you can define, and use in context, the key terms below. It denotes a full s orbital. Web a neutral helium atom, with an atomic number of 2 (z = 2), has two electrons. Web chemistry questions and answers. Electron configurations describe where electrons are located around the nucleus of an atom. Therefore, the electron configuration for a neutral atom of helium is 1s2. Web for hydrogen, therefore, the single electron is placed in the 1 s orbital, which is the orbital lowest in energy (figure 2.7.1 2.7. Web the electron configuration of helium is 1s 2 , if the electron arrangement is through orbitals. Energy 1 i x $ ? Draw. Draw the electron configuration for a neutral atom of helium. Both electrons fit into the 1s subshell because s subshells contain one s orbital which can hold up to 2 electrons; You'll get a detailed solution from a subject matter expert that helps you learn core concepts. This means that the electron configuration for helium has to. Draw the electron. The first electron has the same four quantum numbers as the hydrogen atom electron (n = 1, l = 0, m l = 0, m s = + 1 2 m s = + 1 2). You'll get a detailed solution from a subject matter expert that helps you learn core concepts. The isotope is defined by the number of. Web helium, #he#, has an atomic number equal to #2#, which means that it has #2# protons in its nucleus. Hydrogen (h) helium (he) lithium (li) beryllium (be) boron (b) carbon (c) nitrogen (n) oxygen (o) fluorine (f) neon (ne) sodium (na) magnesium (mg) aluminum (al) silicon (si. Web chemistry questions and answers. Therefore its electron configuration is, 1s2. Draw. Therefore, the electron configuration for a neutral atom of helium is 1s2. Web the upper right side shows the number of electrons in a neutral atom. Enerov draw the electron configuration for a neutral atom of helium. Electron configuration through orbit (bohr principle) electron configuration through orbital (aufbau principle) helium atom (bohr model) electron configuration through orbitals follows different principles.. Web the electron configuration of helium is 1s2, which means there are two electrons in the 1s orbital. Draw the electron configuration for a neutral atom of beryllium. It denotes a full s orbital. This problem has been solved! Web to write the electron configuration of an atom, identify the energy level of interest and write the number of electrons. Electron configurations describe where electrons are located around the nucleus of an atom. A neutral helium atom will thus have #2# electrons surrounding its nucleus. Draw the electron configuration for a neutral atom of helium. Draw the electron configuration for a neutral atom of beryllium. Web for hydrogen, therefore, the single electron is placed in the 1 s orbital, which. Therefore, the electron configuration for a neutral atom of helium is 1s2. By knowing the electron configuration of an element, we can predict and explain a great deal of its chemistry. This electron configuration calculator will instantly show you the distribution of electrons in the orbitals of any periodic element you choose. This problem has been solved! Both electrons fit. Draw a lewis electron dot diagram for an atom or a monatomic ion. Therefore its electron configuration is, 1s2. Hydrogen (h) helium (he) lithium (li) beryllium (be) boron (b) carbon (c) nitrogen (n) oxygen (o) fluorine (f) neon (ne) sodium (na) magnesium (mg) aluminum (al) silicon (si. Web the electron configuration of helium is 1s 2 , if the electron. Web draw the electron configuration for a neutral atom of helium. Web this structure is called an electron configuration and is unique to hydrogen. Draw the electron configuration for a neutral atom of helium. This problem has been solved! Web a neutral helium atom, with an atomic number of 2 (z = 2), has two electrons. The isotope is defined by the number of neutrons in an atom, which might be equal to the number of protons—or not. The helium atom contains two protons and two electrons. This means that the electron configuration for helium has to. Web helium, #he#, has an atomic number equal to #2#, which means that it has #2# protons in its nucleus. Remember, a neutral atom contains the same number of protons and electrons. This is the electron configuration of helium; Web the electron configuration of helium is 1s 2 , if the electron arrangement is through orbitals. Hydrogen (h) helium (he) lithium (li) beryllium (be) boron (b) carbon (c) nitrogen (n) oxygen (o) fluorine (f) neon (ne) sodium (na) magnesium (mg) aluminum (al) silicon (si. Make certain that you can define, and use in context, the key terms below. For example, the electron configuration of lithium, 1s²2s¹, tells us that lithium has two electrons in the 1s subshell and one electron in the 2s subshell. Energy 1 l х 5 ? Therefore its electron configuration is, 1s2. Web a neutral helium atom, with an atomic number of 2 (z = 2), has two electrons. Web chemistry questions and answers. Draw a lewis electron dot diagram for an atom or a monatomic ion. This problem has been solved!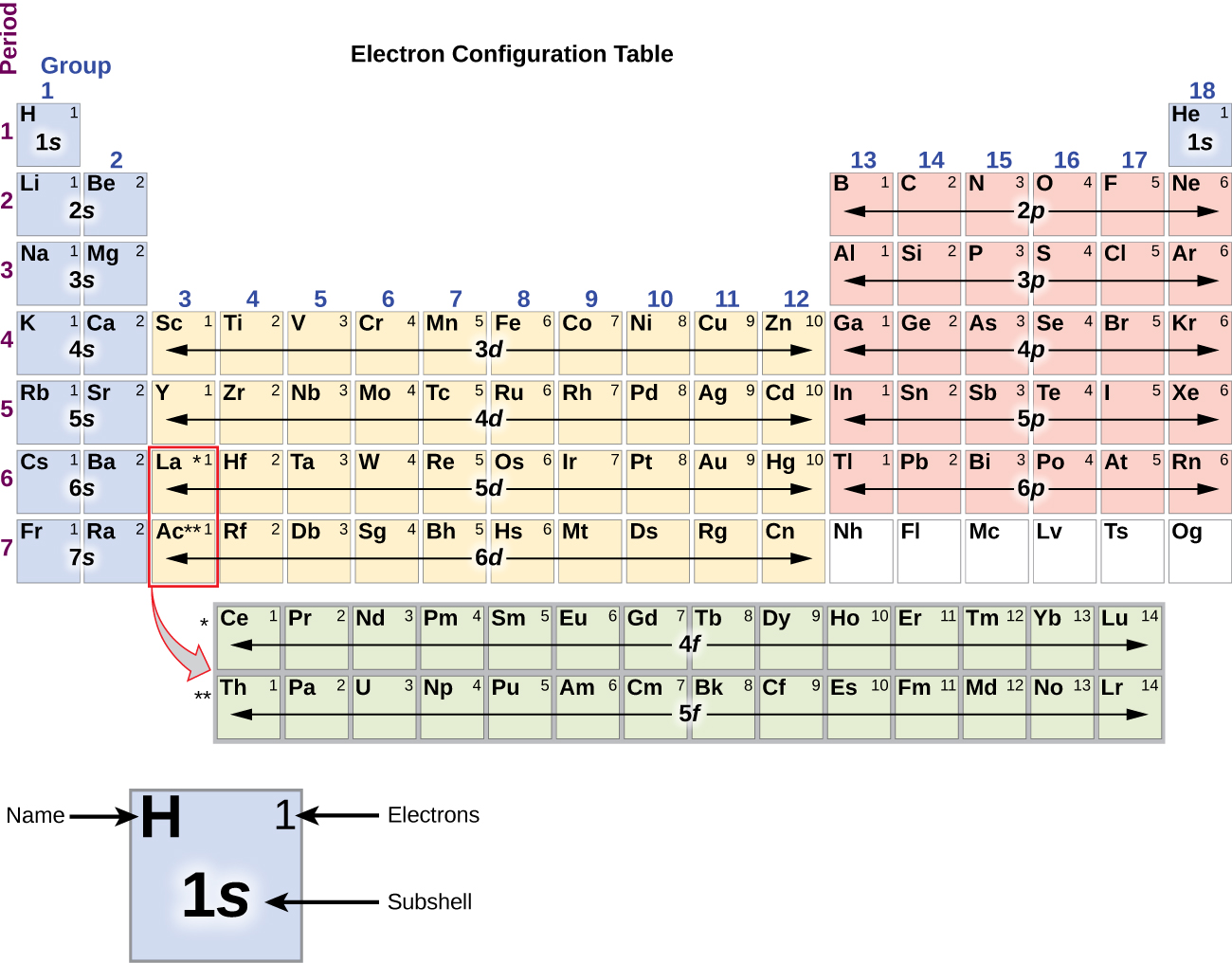
Helium electric configuration saildop
Atom drawing pikolbuyers

Helium, atomic structure Stock Image C018/3683 Science Photo Library
Solved Draw the electron configuration for a neutral atom of
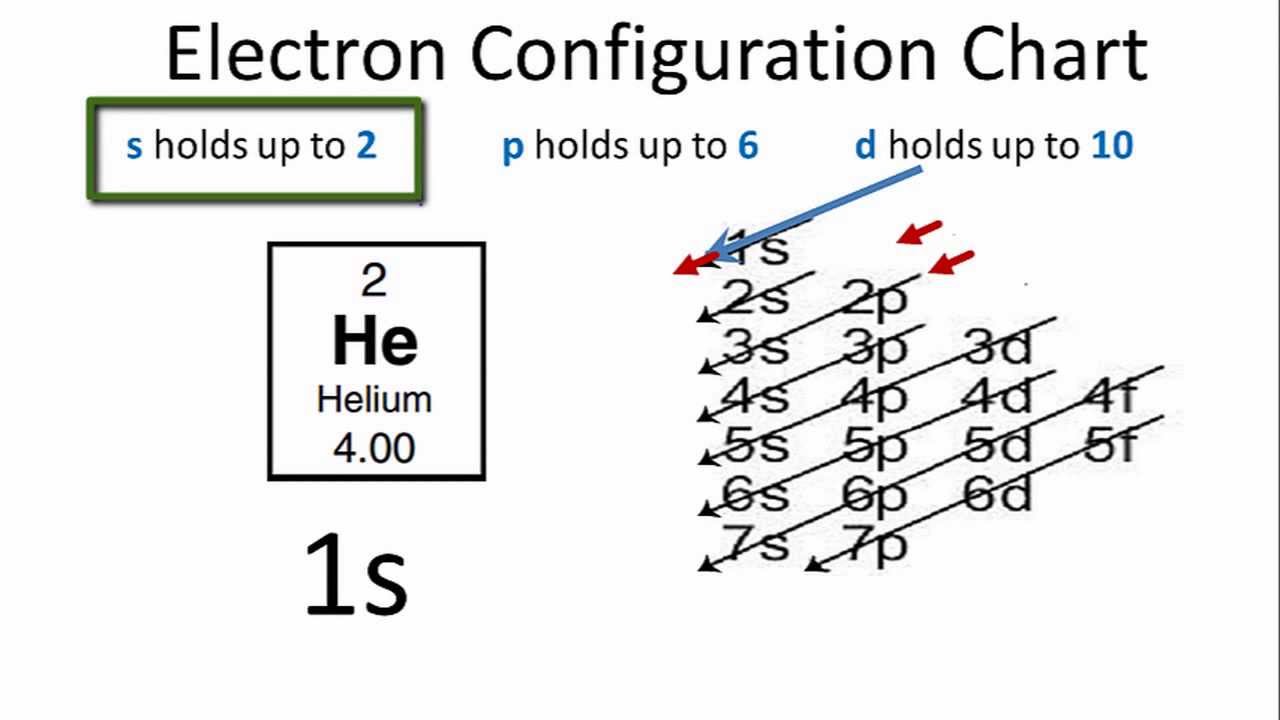
How To Find the Helium Electron Configuration (He)

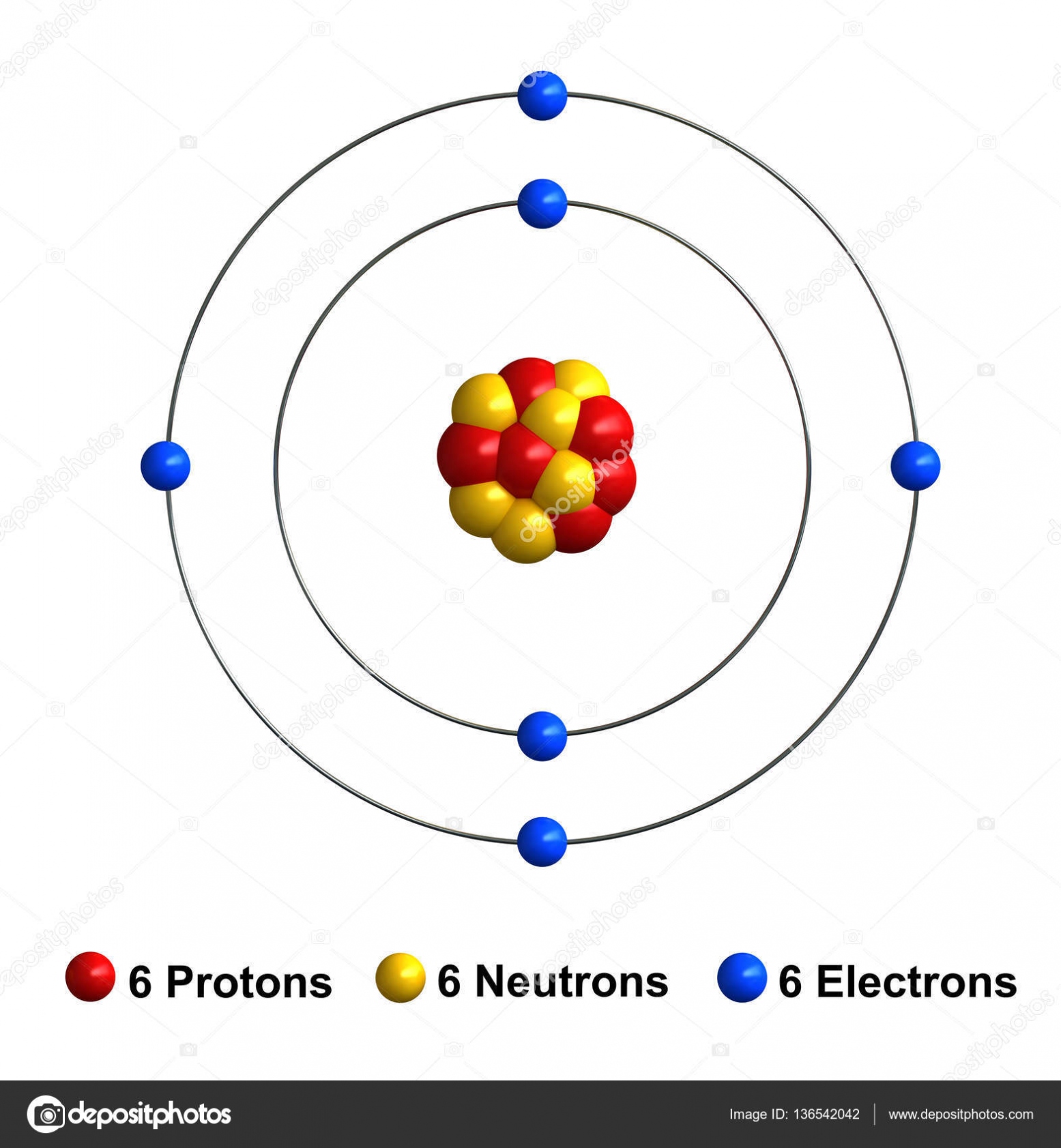
Helium Atom Diagram
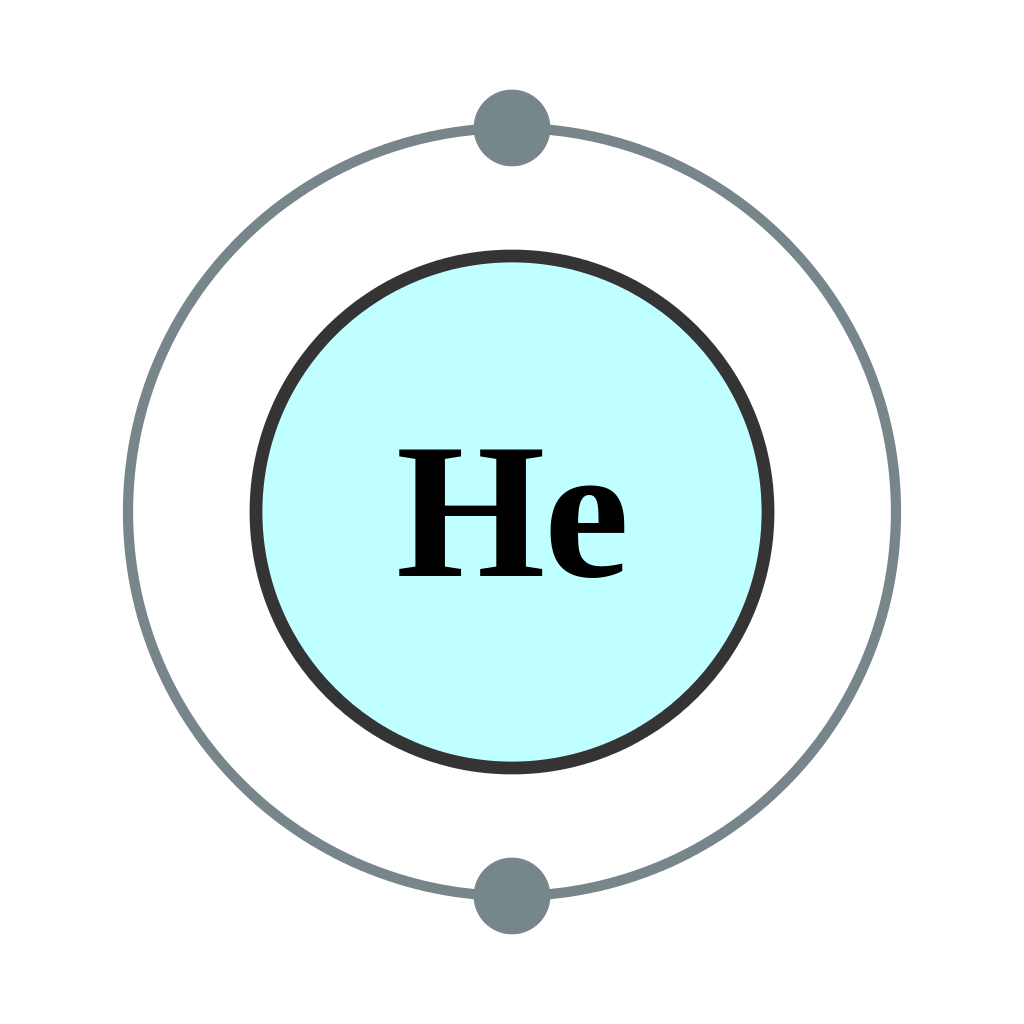
How To Find the Helium Electron Configuration (He)
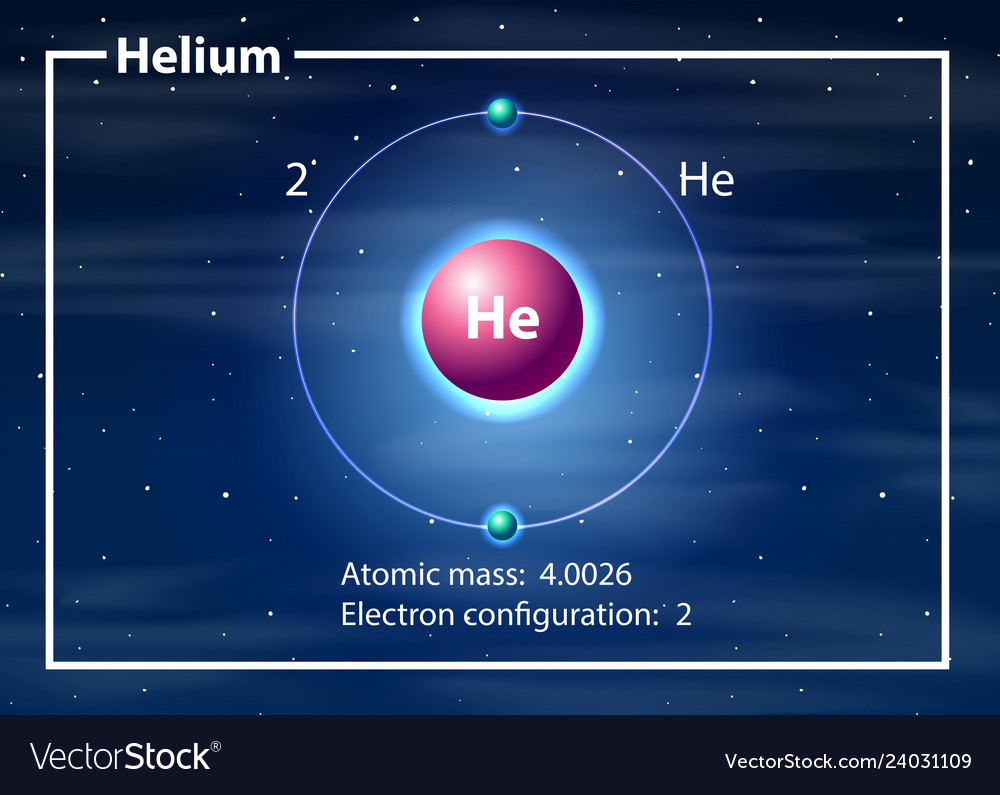
Helium atom diagram concept Royalty Free Vector Image
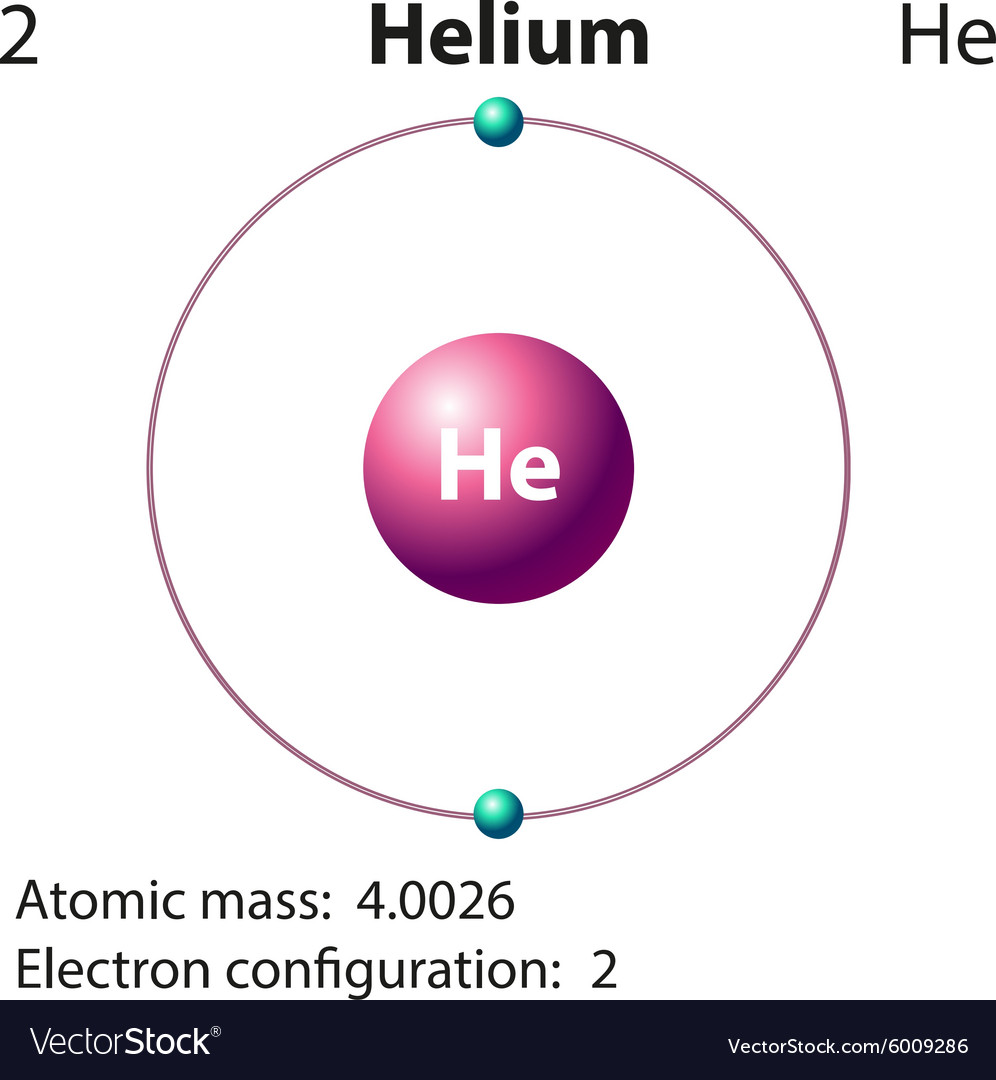
Diagram representation of the element helium Vector Image
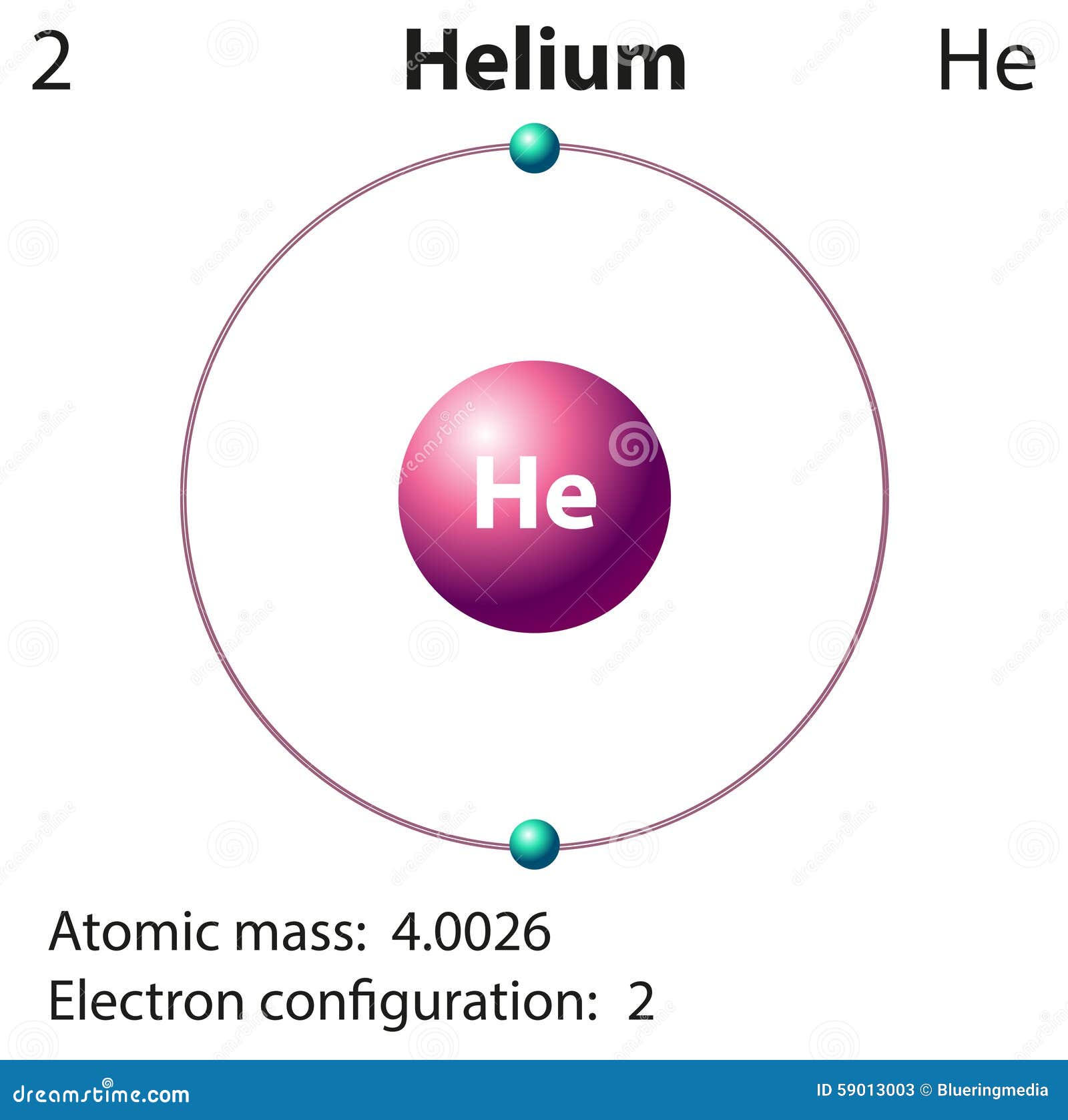
Diagram Representation of the Element Helium Stock Vector
Draw The Electron Configuration For A Neutral Atom Of Helium.
Web The Electron Configuration Of An Element Is The Arrangement Of Its Electrons In Its Atomic Orbitals.
Web To Write The Electron Configuration Of An Atom, Identify The Energy Level Of Interest And Write The Number Of Electrons In The Energy Level As Its Superscript As Follows:
Want To Join The Conversation?
Related Post: