Draw A Second Resonance Form For The Structure Shown Below
Draw A Second Resonance Form For The Structure Shown Below - Draw a second resonance form for the structure shown below: Web identify the positions of the atoms and the location of the pi bonds and lone pairs in the given structure to determine which electrons can be delocalized in creating a new. Web draw the new resonance structure: 1) there is only one real structure for each molecule or. Web draw a second resonance form for the structure shown below. Web choose the most favorable lewis structure. Draw a second resonance form for the structure shown below. Draw a second resonance form for the structure shown. Equivalent lewis structures are called resonance forms. Web a resonance form is another way of drawing a lewis dot structure for a given compound. The structure shown below has two possible resonance forms, which are: Web draw the new resonance structure: The first resonance form would be: The second resonance form would be: Next, add the first peak. Start with the structure shown: 1) there is only one real structure for each molecule or. Web when learning to draw and interpret resonance structures, there are a few basic guidelines to help. Equivalent lewis structures are called resonance forms. This problem has been solved! Web draw the second resonance form for the structure shown below. This problem has been solved! Draw a second resonance form for the structure shown below. Draw a second resonance form for the structure shown. • include all valence one pairs in your answer. 1) there is only one real structure for each molecule or. The structure shown below has two possible resonance forms, which are: Draw the resonance structures of molecules or ions that exhibit. Draw a second resonance form for the structure shown below. Web step 1/2 first, we need to identify the atoms that can move their electrons to form a. Web draw a second resonance form for the structure this problem has been solved! After completing this section, you should be able to. Draw a second resonance form for. The second resonance form would be: Submitted by dorothy p., oct. Start with the structure shown: Draw a second resonance form for the structure shown below. Draw a second resonance form for. Web draw the second resonance form for the structure shown below. The structure shown below has two possible resonance forms, which are: Start with the structure shown: Web draw the second resonance form for the structure shown below. • include all valence lone. Web identify the positions of the atoms and the location of the pi bonds and lone pairs in the given structure to determine which electrons can be delocalized in creating a new. • include all valence one pairs in. 1) there is only one real structure for each molecule or. Draw the resonance structures of molecules or ions that exhibit. Web draw a second resonance form for the structure shown below. Web when learning to draw and interpret resonance structures, there are a few basic guidelines to help. Web draw a second resonance form for the structure this problem. Web the second resonance form for the structure shown below is: Draw a second resonance form for the structure shown below: To generate the structure shown below, start by drawing the base structure. Draw a second resonance form for the structure shown below. After completing this section, you should be able to. H2c ch2 draw a second resonance form. Web choose the most favorable lewis structure. Equivalent lewis structures are called resonance forms. The new resonance structure will have a double bond between the carbon and hydrogen, and a single bond between the carbon and. Start with the structure shown: Web a resonance form is another way of drawing a lewis dot structure for a given compound. Web choose the most favorable lewis structure. Web the second resonance form for the structure shown below is: Web draw a second resonance form for the structure shown below. Web draw the new resonance structure: Equivalent lewis structures are called resonance forms. Web draw a second resonance form for the structure this problem has been solved! The structure shown below has two possible resonance forms, which are: Draw a second resonance form for. Become a study.com member to unlock this answer! Draw a second resonance form for the structure shown below. Draw a second resonance form for the structure shown below: The first resonance form would be: In this case, we have a nitrogen atom with a lone pair and a carbon. You'll get a detailed solution from a subject matter expert that helps you learn. The new resonance structure will have a double bond between the carbon and hydrogen, and a single bond between the carbon and.
OneClass Draw a second resonance form for the structure shown below
[Solved] Draw a second resonance form for the structure shown below
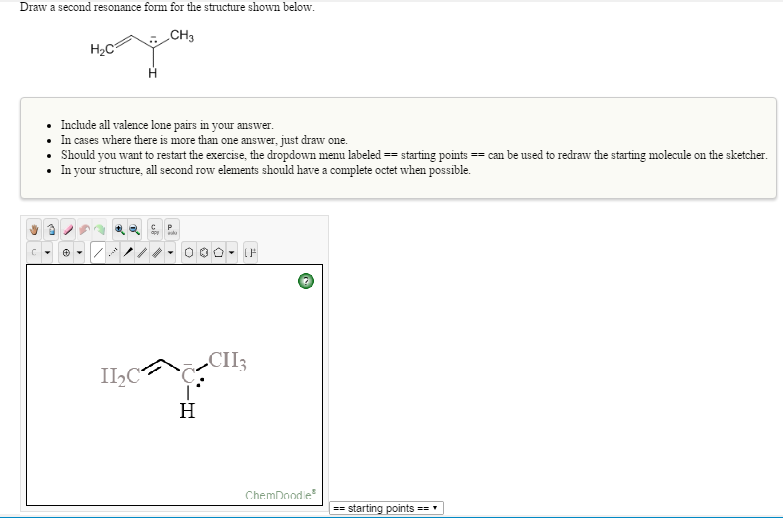
Solved Draw a second resonance form for the structure shown
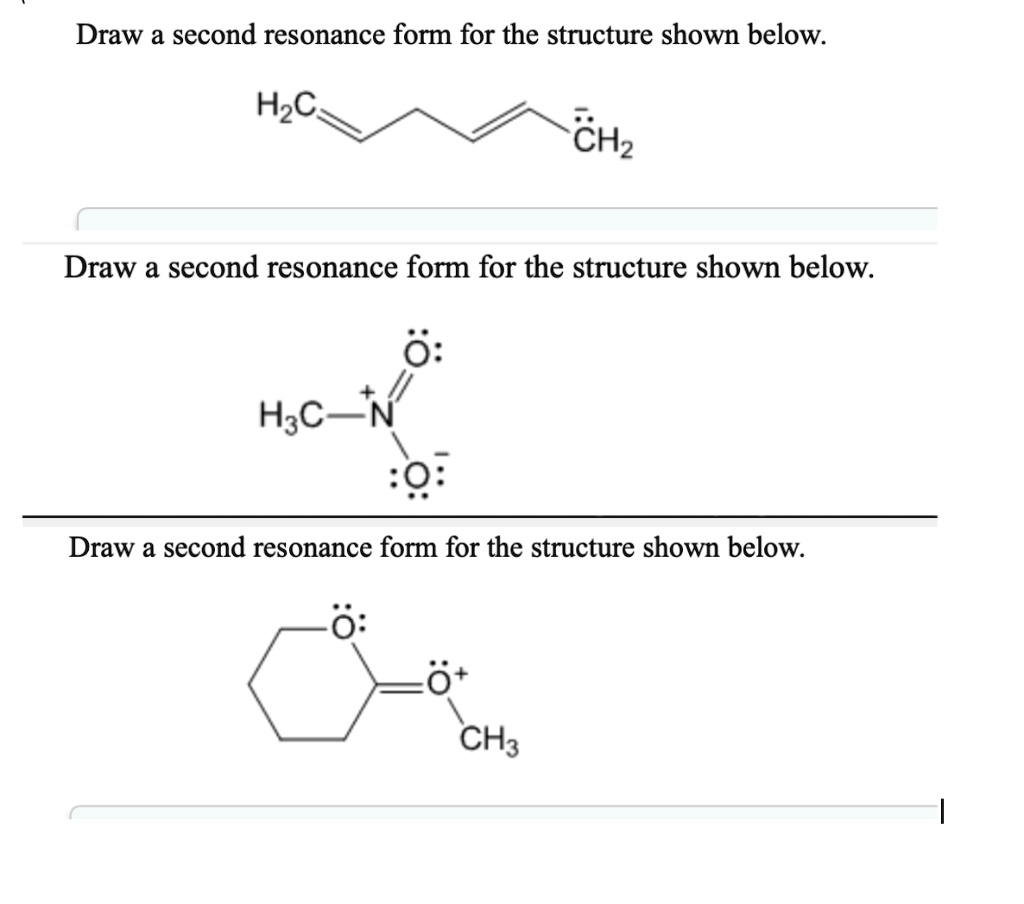
SOLVED Draw a second resonance form for the structure shown below H3C
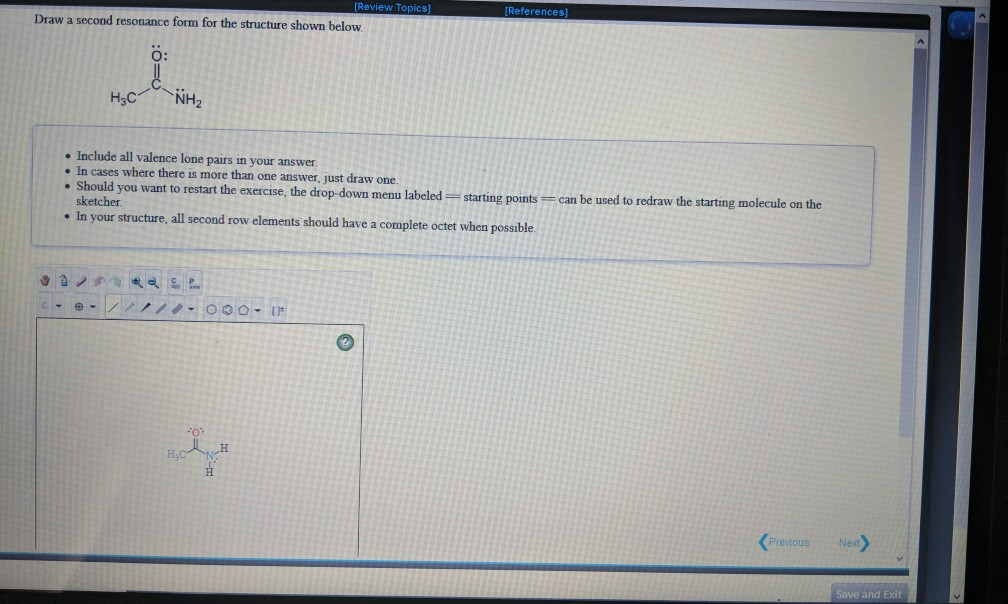
[Solved] Draw a second resonance form for the structure shown below. H
[Solved] Draw a second resonance form for the structure shown below. O

Solved Draw a second resonance form for the structure shown

OneClass Draw a second resonance form for the structure shown below
Solved Draw a second resonance form for the structure shown
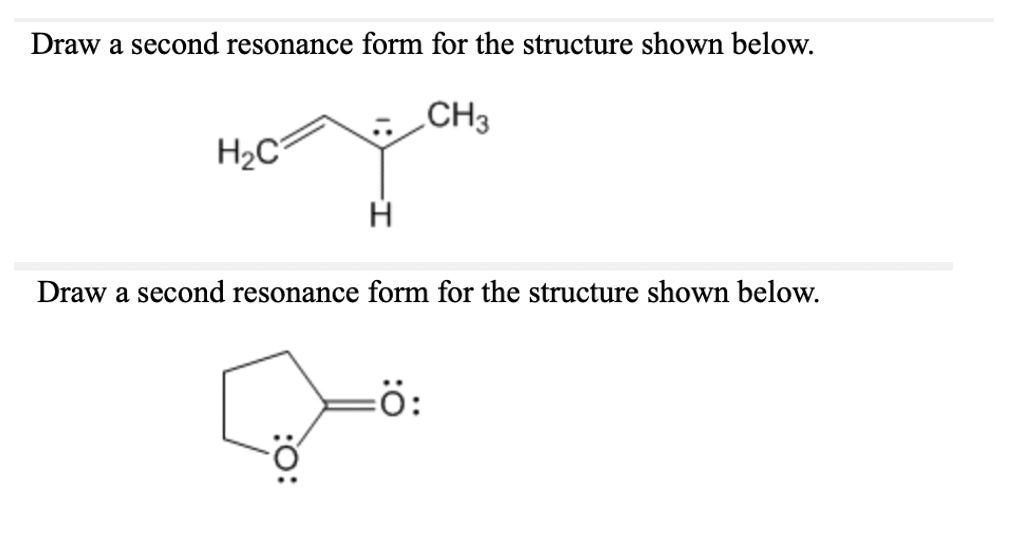
SOLVED Draw a second resonance form for the structure shown below CH3
Web You'll Get A Detailed Solution From A Subject Matter Expert That Helps You Learn Core Concepts.
Draw A Second Resonance Form For The Structure Shown Below.
Web Step 1/2 First, We Need To Identify The Atoms That Can Move Their Electrons To Form A Double Bond Or A Lone Pair.
After Completing This Section, You Should Be Able To.
Related Post: