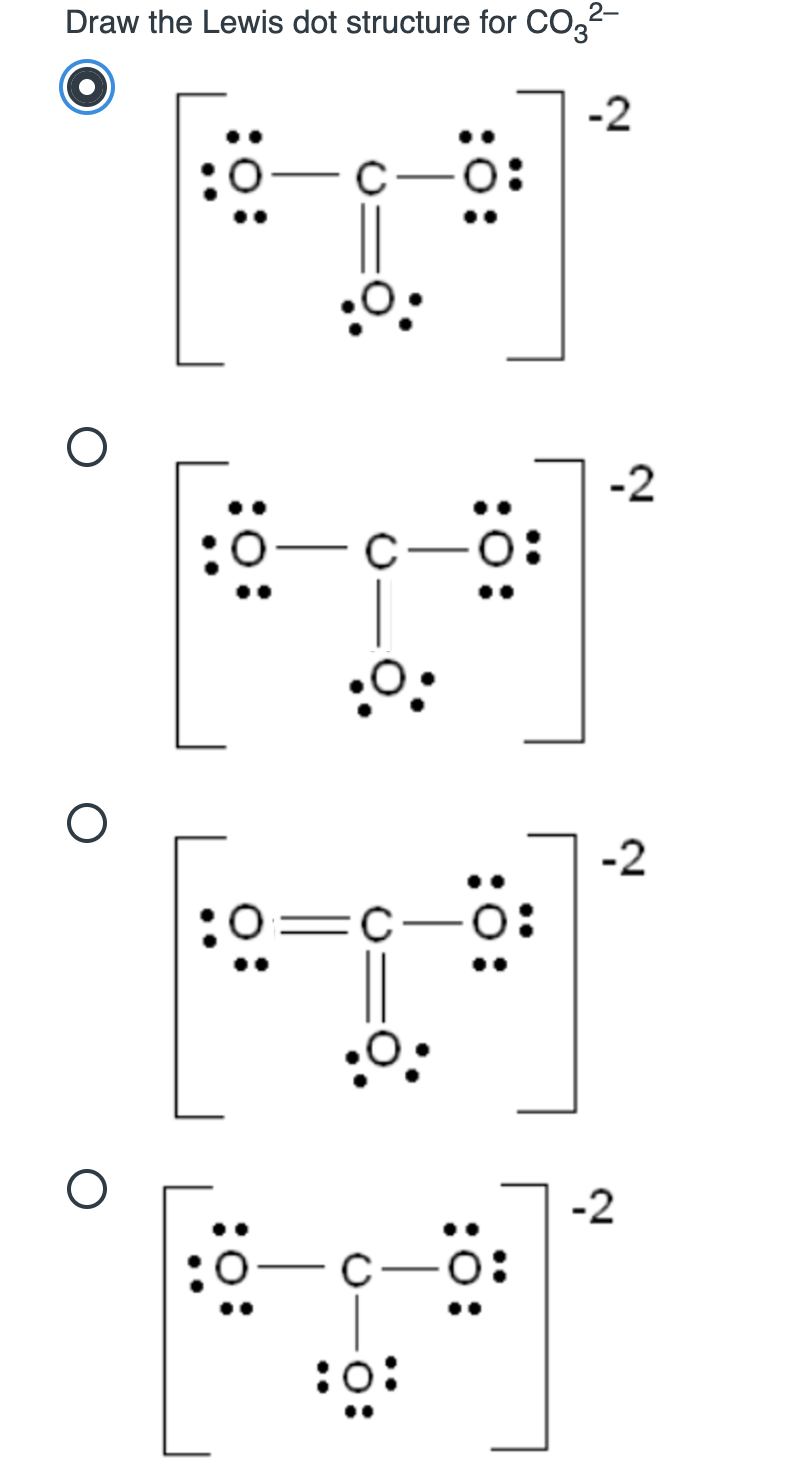
Draw A Lewis Structure For Co32
Draw A Lewis Structure For Co32 - Valence electrons are the electrons in the outermost shell of an atom that participate in chemical bonding. 31k views 1 year ago. This video discusses the resonance. Web drawing lewis structures for molecules with one central atom: The number of unshared pairs (lone pairs) on the central catom is: Web lewis structure is the name given to such a skeletal diagram where we use the symbols of the atoms and use dots to represent the valence shell electrons. Using lewis electron structures to explain stoichiometry. I also go over the resonance, hybridization, shape and bond angle. Then, determine the formal charges on the atoms and minimize them by converting any available lone pairs to chemical bonds. Obeys the octet rule b. This problem has been solved! Hence, lewis structure is also commonly called electron dot structure. Therefore it is put in the center of the dot structure. Take a pen and paper with you and try to draw this lewis structure along with me. (assign lone pairs, radical electrons, and atomic charges where appropriate.) calculate the electrons required (er), valence electrons. This video discusses the resonance. The lewis structure for co2− 3 is shown in the figure. The central catom forms double bonds. Which of the following statements is true? Click the card to flip 👆. Web draw the lewis structure of the carbonate ion, co 32−. Not the question you’re looking for? The lewis structure for co2− 3 is shown in the figure. For the central carbon atom: The central catom forms double bonds. Valence electrons are the electrons in the outermost shell of an atom that participate in chemical bonding. You'll get a detailed solution from a subject matter expert that helps you learn. Draw the lewis dot structure of the following. (assign lone pairs, radical electrons, and atomic charges where appropriate.) calculate the electrons required (er), valence electrons (ve), and shared pairs. 31k views 1 year ago. Let’s draw and understand this lewis dot structure step by step. Web 174k views 3 years ago. Find total number of electrons of the valance shells of carbon and oxygen atoms. Web draw the lewis structure of the carbonate ion, co 32−. Hence, lewis structure is also commonly called electron dot structure. You'll get a detailed solution from a subject matter expert that helps you learn. 31k views 1 year ago. Count the total number of. Web draw the lewis structure of the carbonate ion, co 32−. The central catom forms single bonds. Which of the following statements is true? You'll get a detailed solution from a subject matter expert that helps you learn. Valence electrons are the electrons in the outermost shell of an atom that participate in chemical bonding. I also go over the resonance, hybridization, shape and bond angle. In the lewis structure, the outermost orbit electrons of each atom is shown. Web drawing lewis structures for molecules with one central atom: The central catom forms single bonds. Put lone pairs on atoms. Not the question you’re looking for? Draw the lewis structure for co2− 3. Count the total number of. Take a pen and paper with you and try to draw this lewis structure along with me. Valence electrons are the electrons in the outermost shell of an atom that participate in chemical bonding. The lewis structure for co2− 3 is shown in the figure. Find total number of electrons of the valance shells of carbon and oxygen atoms. 592k views 10 years ago. You'll get a detailed solution from a subject matter expert that helps you learn. Hence, lewis structure is also commonly called electron dot structure. Count the total number of. The central catom forms single bonds. This video discusses the resonance. Co2− 3, h clo4, h n o3. Using formal charges to determine how many bonds to make, a different perspective. The central catom forms double bonds. Not the question you’re looking for? Draw the lewis dot structure of the following. Here’s the best way to solve it. The lewis structure for co2− 3 is shown in the figure. Web draw the lewis structure of the carbonate ion, co 32−. Web the carbon atom (c) is at the center and it is surrounded by 3 oxygen atoms (o). (assign lone pairs, radical electrons, and atomic charges where appropriate.) calculate the electrons required (er), valence electrons (ve), and shared pairs (sp). Then, determine the formal charges on the atoms and minimize them by converting any available lone pairs to chemical bonds. Click the card to flip 👆. This problem has been solved! Let’s draw and understand this lewis dot structure step by step.Solved Draw the Lewis dot structure for CO32 2 0 O 2 0

CO32 Lewis Structure (Carbonate Ion) YouTube
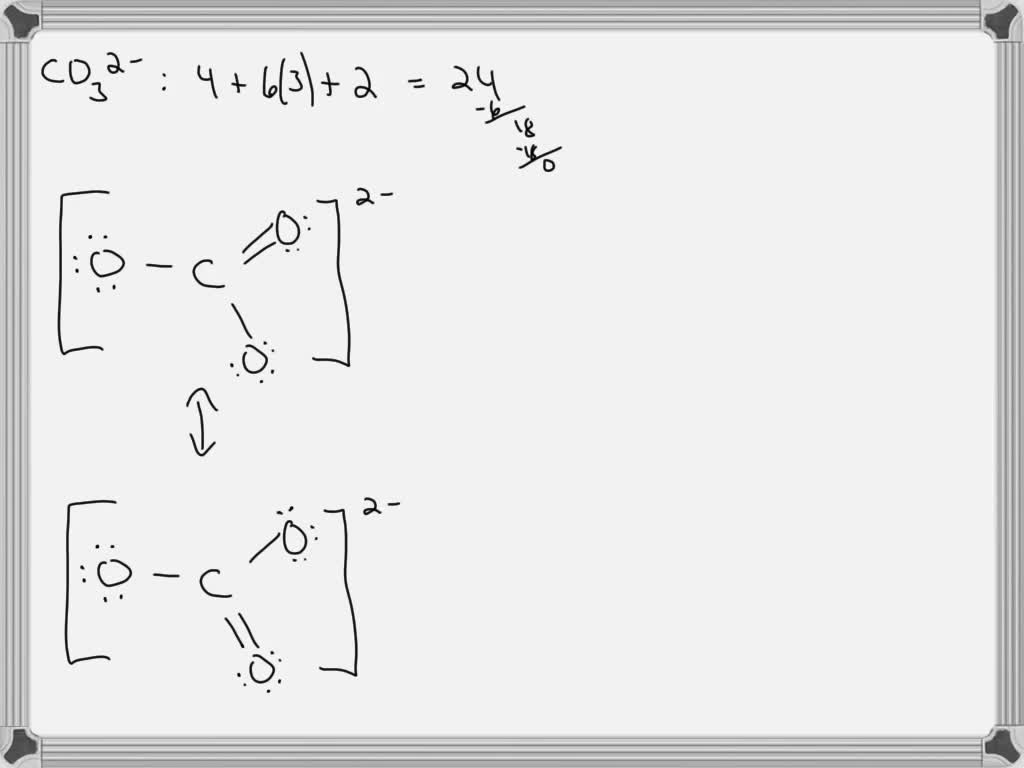
SOLVED For CO32 , carbonate ion, draw the Lewis structure (by

How To Draw The Lewis Structure of CO3 2 (Carbonate Ion) Chemistry

Lewis Structure for CO3 2 (Carbonate ion) YouTube
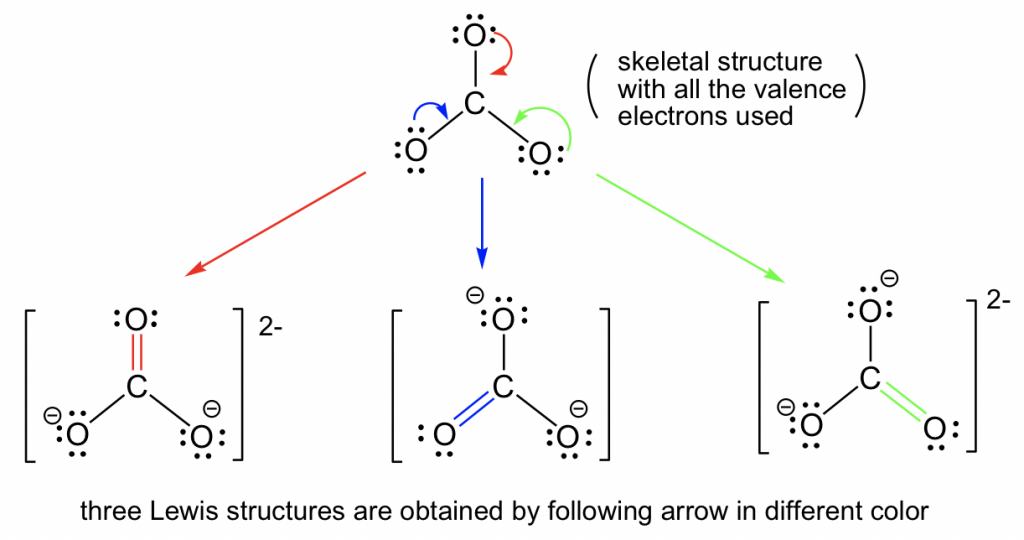
1.3 Resonance Structures Chemistry LibreTexts

CO32 Lewis Structure How to Draw the Lewis Structure for CO3 2

How to draw the Lewis structure of CO3 2 (Carbonate ion) YouTube
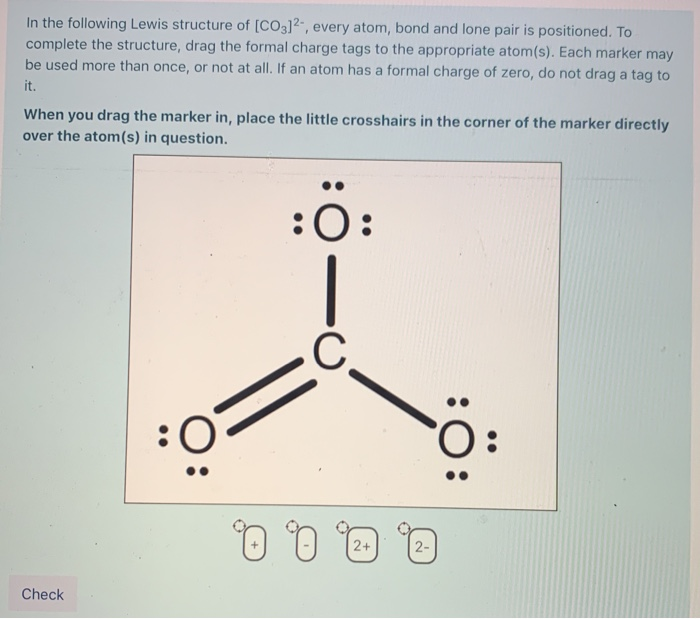
Solved In the following Lewis structure of [CO3)2, every

Draw the Lewis Structure for Co32
Web Lewis Structure Is The Name Given To Such A Skeletal Diagram Where We Use The Symbols Of The Atoms And Use Dots To Represent The Valence Shell Electrons.
Breaking The Octet Rule ;
In The Lewis Structure, The Outermost Orbit Electrons Of Each Atom Is Shown.
Web 174K Views 3 Years Ago.
Related Post: