Draw A Lewis Diagram For C2H2
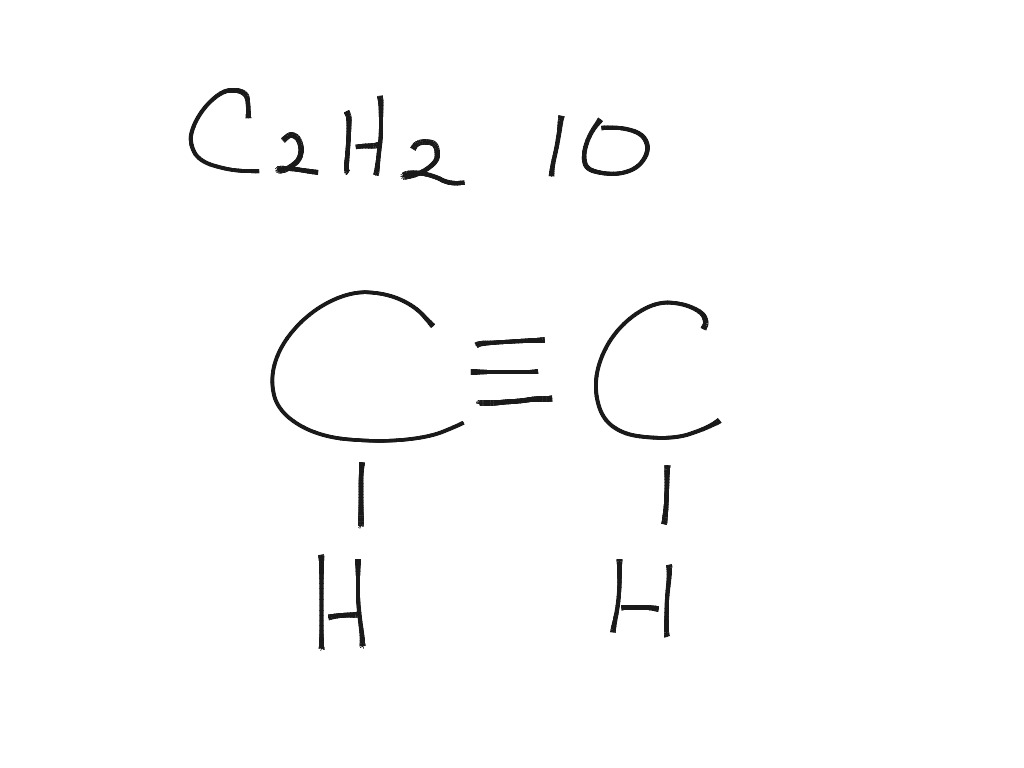
Draw A Lewis Diagram For C2H2 - Determine the electron and molecular group geometry for each structure. Add together the valence electrons from each atom. (1 pts) h h h h h 8. What is the hybridization of the central atom in c2h2 d. Web how to draw the lewis dot structure for c2h2: #2 mark lone pairs on the atoms. Is it polar or nonpolar ? This structure is essential in understanding the properties and behavior of. Find the total valence electrons in c2h2 molecule. Include all lone pairs of electrons.draw the lewis structure for c2h4 (whose skeletal structure is h2cch2 ). 176k views 12 years ago every video. Draw the molecule by placing atoms on the grid and connecting them with bonds. Web chemistry questions and answers. Web to draw a lewis structure of any molecule and understand the bond formation in any molecule, it is essential to know the total number of valence electrons. #1 first draw a rough sketch. This structure is essential in understanding the properties and behavior of. The following procedure can be used to draw lewis structure for simple molecules. Valence electrons of carbon atom +. Include all lone pairs of electrons.draw the lewis structure for c2h4 (whose skeletal structure is h2cch2 ). 176k views 12 years ago every video. We experienced a large temperature swing during a softball. Web draw lewis structures for covalent compounds. Find the total valence electrons for the c2h2 molecule. Is it polar or nonpolar ? Web there are several steps to draw a lewis structure of a molecule. I quickly take you through how to draw the lewis structure of chch (acetylene or ethyne). #2 mark lone pairs on the atoms. Determine the total number of valence electrons in the molecule or ion. Web here are the steps to draw the c2h2 lewis structure in an active voice and concise manner: Lewis structure of c2h2 (or acetylene or. Valence electrons of carbon atom +. Is it polar or nonpolar ? Draw the lewis structure for c2h2 (whose skeletal structure is hcch ). Find the total valence electrons for the c2h2 molecule. Those steps are used in detail in this tutorial to draw c 2 h 2 lewis structure. Use these steps to correctly draw the c 2 h 2 lewis structure: #3 calculate and mark formal charges on the atoms, if required. Valence electrons of carbon atom +. Draw the molecule by placing atoms on the grid and connecting them with bonds. You can see how the sp hybridized orbitals combine and overlap to form a bonding sigma. Determine the total number of valence electrons in the molecule by adding the valence electrons of each atom. Lewis structures are representations of molecules that include not only what atoms are present in the molecule but also how the atoms are connected. Lewis structures show all of the valence electrons in an atom or molecule. Web how to draw the. Web draw lewis structures for covalent compounds. Try to draw the c 2 h 2 lewis structure before watching the video. In order to find the total valence electrons in c2h2 molecule, first of all you should know the valence electrons present in carbon atom as well as hydrogen atom. Web how to draw the lewis dot structure for c2h2:. Draw a lewis structure for c2h2 and answer the following questions: For c2h2, the total number of valence electrons is 10. Draw the molecule by placing atoms on the grid and connecting them with bonds. Those steps are used in detail in this tutorial to draw c 2 h 2 lewis structure. Web chemistry questions and answers. The total count of electrons can be known by adding individual atom electrons of c 2 h 2 in the valance shell. (1 pts) h h h h h 8. With c 2 h 2 you are going to run out of valence electrons and will have to share more than one pair of electrons between the carbon atoms. Watch. However, you can learn basic examples of drawing lewis structures. Web learn the steps to draw the lewis structure of c2h2 (ethyne or acetylene) in just 1 minute.📌you can draw any lewis structures by following the simple steps. Find the total valence electrons in c2h2 molecule. Determine the electron and molecular group geometry for each structure. Use these steps to correctly draw the c 2 h 2 lewis structure: Because c 2 h 2 molecule is a simple molecule, those all steps may not be used. Acetylene (ethyne) it is helpful if you: #3 calculate and mark formal charges on the atoms, if required. Remember that hydrogen (h) atoms always go on the outside of a lewis structure. For c2h2, the total number of valence electrons is 10. #2 mark lone pairs on the atoms. The following procedure can be used to construct lewis electron structures for simple molecules. You can see how the sp hybridized orbitals combine and overlap to form a bonding sigma (σ) orbital and an. Try to draw the c 2 h 2 lewis structure before watching the video. Find the total valence electrons for the c2h2 molecule. Web steps of drawing c2h2 lewis structure.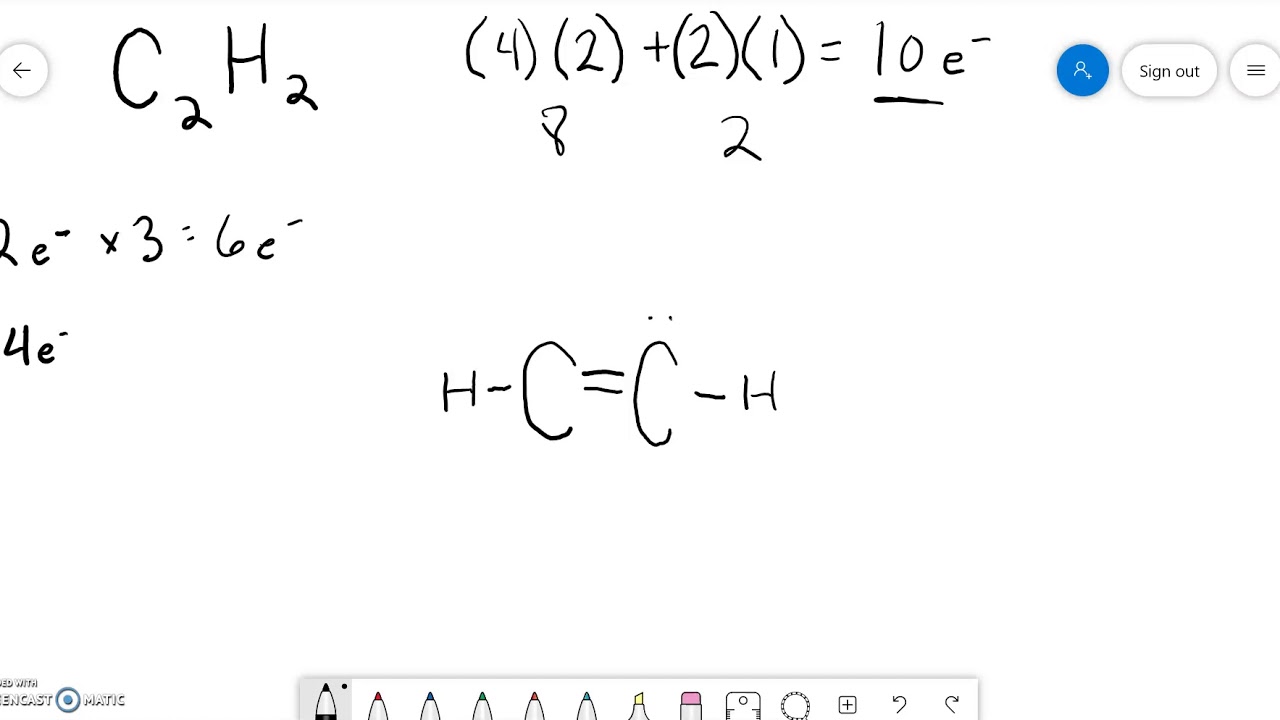
Lewis Structure C2H2 YouTube

Estrutura De Lewis C2h2

C2H2 Lewis Structure, Molecular Geometry, Hybridization, Polarity, and

Estrutura De Lewis C2h2

Lewis electrondot structure of C2H2 YouTube

Lewis Dot Diagram For C2h2
C2h2 Lewis Structure

C2H2 Lewis Structure (Ethyne or Acetylene) Math, Lewis, Molecules

How do you draw the Lewis structure for C2H2? Ethyne or Acetylene

How to Draw the Lewis Dot Structure for C2H2 Acetylene (Ethyne) YouTube
Draw A Lewis Structure For C2H2 And Answer The Following Questions:
(1 Pts) H H H H H 8.
Calculate The Total Number Of Electrons.
Lewis Structure Of C2H2 (Or Acetylene Or Ethyne) Contains One Triple Bond Between The Two Carbon (C) Atoms And Two Single Bonds Between Carbon (C) &.
Related Post: