Draw 3Methylcyclobutanol
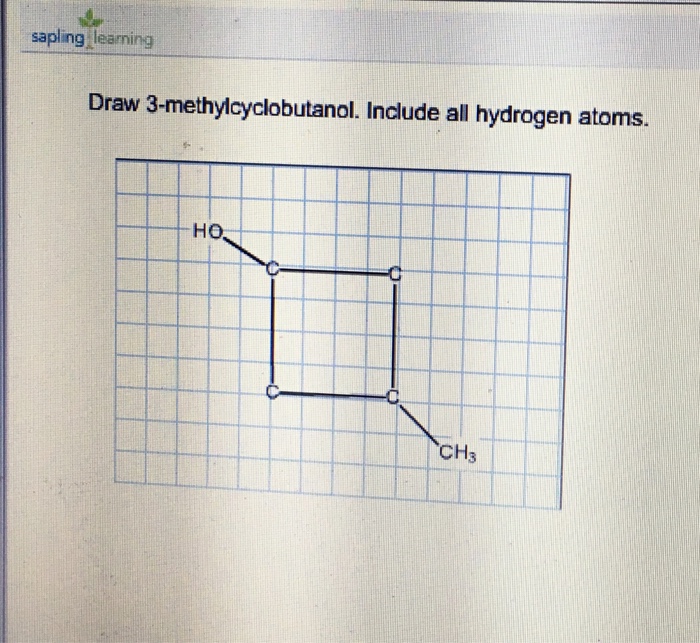
Draw 3Methylcyclobutanol - Make sure that all carbons have four bonds and all hydrogens have one. Molecular formula c 5 h 10 o; This problem has been solved! Add a methyl group (ch3) to one of the carbons in the ring. Draw rings select || h₂c с o | с h₂ h₂c what is wrong with this? Add a methyl group to one of the carbons in the ring. I'u | ch3 h oh more. Web the international union of pure and applied chemistry (iupac) system is used to name alcohols. Start by drawing a cyclobutane ring, which has four carbons in a ring. This problem has been solved! This problem has been solved! Add a methyl group (ch3) to carbon 3 of the cyclobutane. This problem has been solved! Draw rings select || h₂c с o | с h₂ h₂c what is wrong with this? Web the international union of pure and applied chemistry (iupac) system is used to name alcohols. Web start by drawing a cyclobutane ring, which consists of four carbon atoms in a cyclical structure. Web the international union of pure and applied chemistry (iupac) system is used to name alcohols. I'u | ch3 h oh more. You'll get a detailed solution from a. Make sure that all carbons have four bonds and all hydrogens have one. This problem has been solved! Make sure that all carbons have four bonds and all hydrogens have one. I'u | ch3 h oh more. Draw rings select || h₂c с o | с h₂ h₂c what is wrong with this? Molecular formula c 5 h 10 o; Start by drawing a cyclobutane ring, which has four carbons in a ring. This problem has been solved! You'll get a detailed solution from a. This means that one of the. Web start by drawing a cyclobutane ring, which consists of four carbon atoms in a cyclical structure. Web start by drawing a cyclobutane ring, which consists of four carbon atoms in a cyclical structure. This problem has been solved! Start by drawing a cyclobutane ring, which has four carbons in a ring. Make sure that all carbons have four bonds and all hydrogens have one. Add a methyl group to one of the carbons in the ring. I'u | ch3 h oh more. Add a methyl group (ch3) to one of the carbons in the ring. Draw rings select || h₂c с o | с h₂ h₂c what is wrong with this? This problem has been solved! You'll get a detailed solution from a. You'll get a detailed solution from a. This problem has been solved! Web the international union of pure and applied chemistry (iupac) system is used to name alcohols. This means that one of the. Molecular formula c 5 h 10 o; You'll get a detailed solution from a. Add a methyl group to one of the carbons in the ring. Web the international union of pure and applied chemistry (iupac) system is used to name alcohols. Molecular formula c 5 h 10 o; Add a methyl group (ch3) to one of the carbons in the ring. Add a methyl group (ch3) to carbon 3 of the cyclobutane. Molecular formula c 5 h 10 o; You'll get a detailed solution from a. This problem has been solved! Make sure that all carbons have four bonds and all hydrogens have one. Add a methyl group (ch3) to carbon 3 of the cyclobutane. This problem has been solved! This problem has been solved! Web the international union of pure and applied chemistry (iupac) system is used to name alcohols. Web start by drawing a cyclobutane ring, which consists of four carbon atoms in a cyclical structure. You'll get a detailed solution from a. This problem has been solved! Web the international union of pure and applied chemistry (iupac) system is used to name alcohols. You'll get a detailed solution from a. Make sure that all carbons have four bonds and all hydrogens have one. Molecular formula c 5 h 10 o; This means that one of the. Add a methyl group (ch3) to carbon 3 of the cyclobutane. Start by drawing a cyclobutane ring, which has four carbons in a ring. I'u | ch3 h oh more. This problem has been solved! Draw rings select || h₂c с o | с h₂ h₂c what is wrong with this? Include all the hydrogen atoms.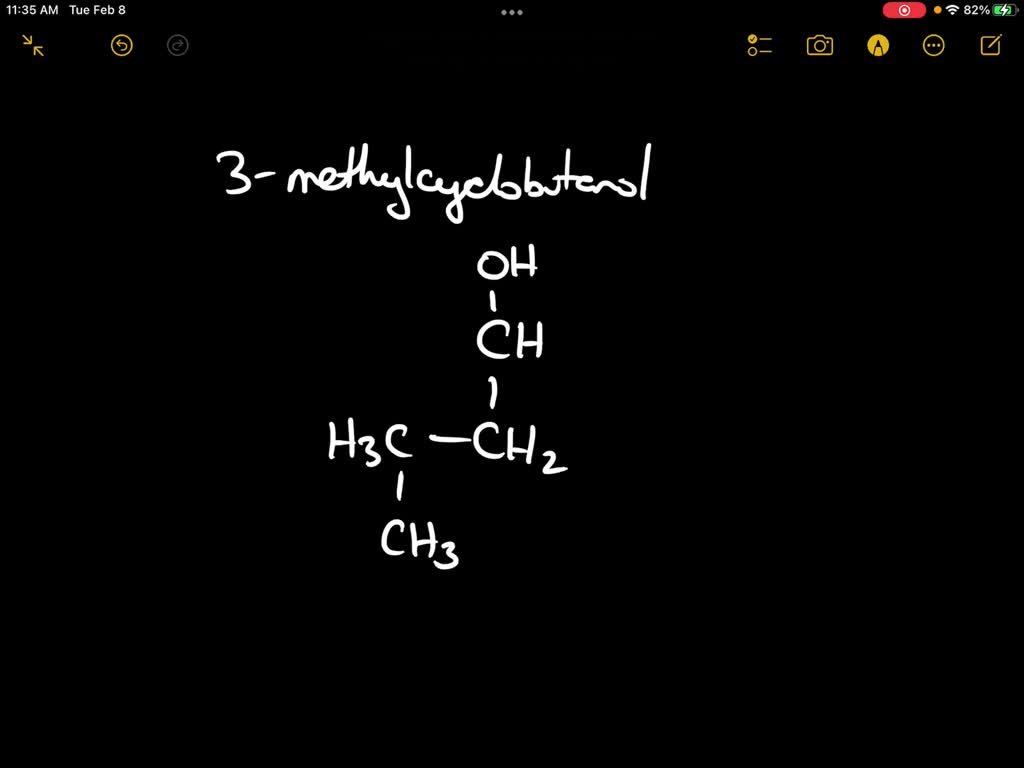
SOLVED Draw 3methylcyclobutanol. Include all hydrogen atoms.

OneClass Draw 3methylcyclobutanol Priceline. m TripAdvisor ã £ã all
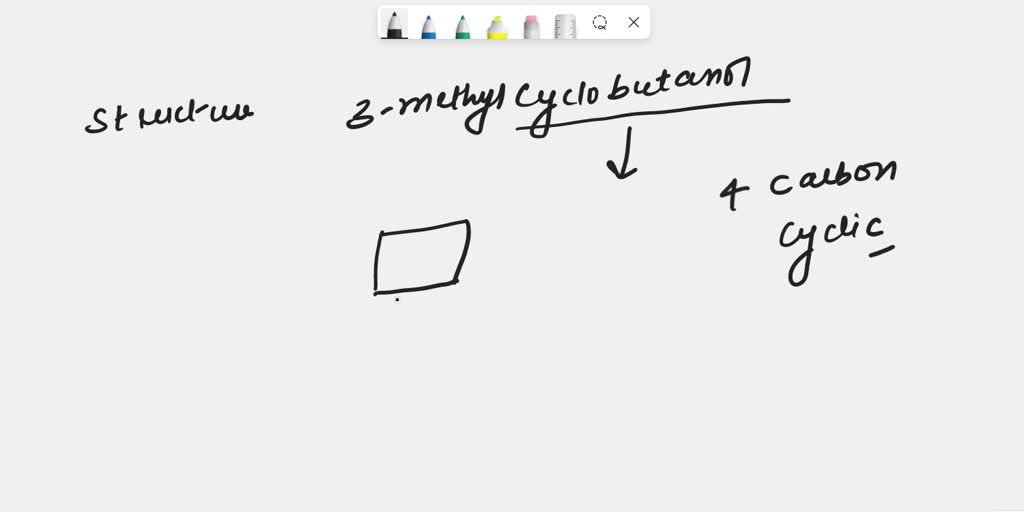
SOLVED Draw 3methylcyclobutanol.
Solved Draw 3methylcyclobutanol. Select Draw Rings More
Solved Sapling Learning Draw 3methylcyclobutanol. Includ...
Solved Draw 3methylcyclobutanol. Include all hydrogen
[Solved] Draw 3methylcyclobutanol. Include all hydrogen atoms. Select
[Solved] Draw 3‑methylcyclobutanol Course Hero
Solved Draw 3methylcyclobutanol. Include all hydrogen
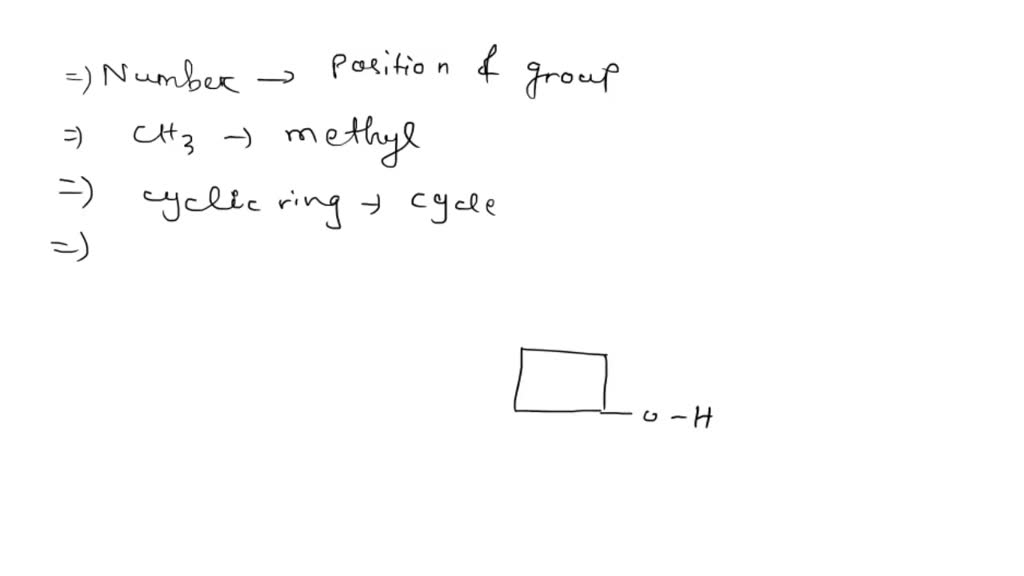
SOLVED Draw 3methylcyclobutanol. Include all hydrogen atoms. Select
Add A Methyl Group To One Of The Carbons In The Ring.
Add A Methyl Group (Ch3) To One Of The Carbons In The Ring.
Web Start By Drawing A Cyclobutane Ring, Which Consists Of Four Carbon Atoms In A Cyclical Structure.
This Problem Has Been Solved!
Related Post: