Draw 10 Water Molecules To Create A Cluster
Draw 10 Water Molecules To Create A Cluster - This weak attraction is often. There are 2 steps to solve this. In the space below draw 10 water molecules to create a cluster be sure to indicate the hydroge. This weak attraction is often referred to as. Web the positive area charge (hydrogen) of one water molecule is attracted to the negative area (oxygen) of a different water molecule. Oo), the hydrogen bond angle (α), and the angle between the proton acceptor plane and the oo linkage (β),. Web below, we'll look at how this hydrogen bonding works. In the space below, draw 10 wate bonds that link the water molecules. Bonds that link the water molecules. Lesson summary the octet rule in covalent bonding covalent compounds are most stable when each atom has eight electrons. In the space below draw 10 water molecules to create a cluster be sure to indicate the hydroge. [1] [2] many such clusters have been predicted by theoretical. Espace below, draw 10 water molecules to create a cluster. This weak attraction is often referred to as. The oxygen atom on one. This weak attraction is often referred to as. Web below, we'll look at how this hydrogen bonding works. Web they happen because the slightly negative oxygen atom of one water molecule is attracted to the slightly positive hydrogen atoms of another water molecule. In the space below draw 10 water molecules to create a cluster be sure to indicate the. Web the positive area charge (hydrogen) of one water molecule is attracted to the negative area (oxygen) of a different water molecule. Web in the space below, draw 10 water molecules to create a cluster. Be sure to indicate the hydrogen. [1] [2] many such clusters have been predicted by theoretical. In the space below, draw 10 wate bonds that. Web a hydrogen bond is formed when the oxygen atom of one water molecule draws the hydrogen atom of another water molecule together in a cluster of water. Web i describe the assembly and characterization of small water clusters on a cu (110) surface in this chapter. The key to understanding water’s chemical behavior is its molecular structure. In the. Extension draw a cluster diagram for each type of bond. Lesson summary the octet rule in covalent bonding covalent compounds are most stable when each atom has eight electrons. This is how a cluster. The key to understanding water’s chemical behavior is its molecular structure. Web below, we'll look at how this hydrogen bonding works. There are 2 steps to solve this. Web updated 8 jul 2022. This weak attraction is often referred to as. In the space below draw 10 water molecules to create a cluster be sure to indicate the hydroge. Be sure to indicate the hydrogen bonds that link the water molecules. Web in chemistry, a water cluster is a discrete hydrogen bonded assembly or cluster of molecules of water. Web the positive area charge (hydrogen) of one water molecule is attracted to the negative area (oxygen) of a different water molecule. Web what i would like to do is to design more water molecules in order to test prof bettens’ method. Bonds that link the water molecules. In the space below, draw 10 wate bonds that link the water molecules. Web what i would like to do is to design more water molecules in order to test prof bettens’ method of fragmentation that computes a chemical system’s electrical. Web in this study, we use computational chemistry to calculate the dynamic equilibrium. Web the positive area charge (hydrogen) of one water molecule is attacted to the negative area (oxygen) of a different water molecule. Be sure to indicate the hydrogen bonds that link the water molecules. Web in this study, a series of functional molecules were added in water to obtain small water cluster systems. Bonds that link the water molecules. In. There are 2 steps to solve this. Web the positive area charge (hydrogen of one water molecule is attracted hydrogen bonding to the negative area (oxygen) of a different water molecule. This weak attraction is often referred to as. Web in this study, a series of functional molecules were added in water to obtain small water cluster systems. Web a. This weak attraction is often referred to as. Web as you read lesson 8.2, use the cluster diagram below to show how each section of the lesson relates to covalent bonding. Web the positive area charge (hydrogen) of one water molecule is attacted to the negative area (oxygen) of a different water molecule. [1] [2] many such clusters have been predicted by theoretical. There are 2 steps to solve this. Bonds that link the water molecules. Web the positive area charge (hydrogen) of one water molecule is attracted to the negative area (oxygen) of a different water molecule. Oo), the hydrogen bond angle (α), and the angle between the proton acceptor plane and the oo linkage (β),. Web they happen because the slightly negative oxygen atom of one water molecule is attracted to the slightly positive hydrogen atoms of another water molecule. This weak attraction is often referred to as. This is how a cluster. Web a hydrogen bond is formed when the oxygen atom of one water molecule draws the hydrogen atom of another water molecule together in a cluster of water. In the space below, draw 10 wate bonds that link the water molecules. Web i describe the assembly and characterization of small water clusters on a cu (110) surface in this chapter. Lesson summary the octet rule in covalent bonding covalent compounds are most stable when each atom has eight electrons. Be sure to indicate the hydrogen bonds that link the water molecules.
(color). Snapshot of a water molecule cluster that forms an

Water cluster design Prof Bettens’ lab The Cut and the Cat

Diagram Of Water Molecule

Condensed concepts Opening the book on water clusters
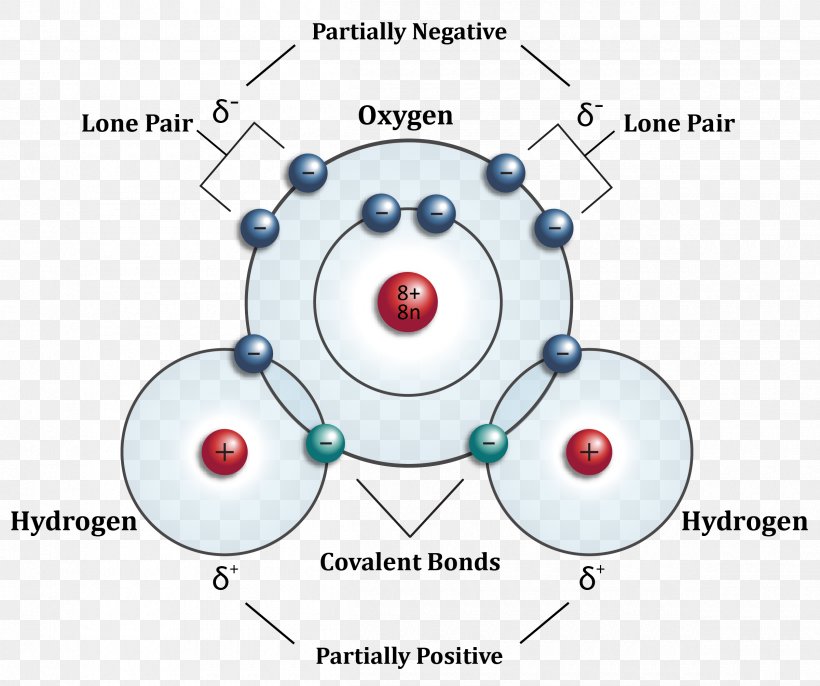
Water Molecular Orbital Diagram

️Water Molecule Worksheet Free Download Goodimg.co

Draw A Diagram Of Water Molecules Labeling The Hydrogen Bond And

Consider the following data concerning the solubility of two gaseous

Water cluster design Prof Bettens’ lab The Cut and the Cat

Water Molecule Hydrogen Bond Diagram
Web The Positive Area Charge (Hydrogen) Of One Water Molecule Is Attracted To The Negative Area (Oxygen) Of A Different Water Molecule.
The Key To Understanding Water’s Chemical Behavior Is Its Molecular Structure.
Web The Positive Area Charge (Hydrogen Of One Water Molecule Is Attracted Hydrogen Bonding To The Negative Area (Oxygen) Of A Different Water Molecule.
Web In Chemistry, A Water Cluster Is A Discrete Hydrogen Bonded Assembly Or Cluster Of Molecules Of Water.
Related Post: