Chemical Bond Drawing
Chemical Bond Drawing - Each hydrogen atom has one bond. 2.1m views 6 years ago new ap & general chemistry video playlist. Each oxygen atom has two bonds. Lewis diagram of the cyanide ion (cn⁻) worked example: Then, compare the model to real molecules! Formal charge and dot structures. Molview consists of two main parts, a structural formula editor and a 3d model viewer. Lewis diagram of xenon difluoride (xef₂) exceptions to the octet rule. We can then combine the valence electrons of different atoms together to form covalent bonds, using a set of. Each nitrogen atom has three bonds. How does molecule shape change with different numbers of bonds and electron pairs? Upload a structure file or draw using a molecule editor. Lewis diagram of the cyanide ion (cn⁻) worked example: When constructing a lewis diagram, keep in mind the octet rule, which refers to the tendency of atoms. Web when drawing the structure of a neutral organic compound,. Web 218,500 structures from the pdb. Web drawing every bond and every atom is tedious, however, so chemists have devised several shorthand ways for writing structures. Web to draw a bond from a single atom, simply click the atom. Molview consists of two main parts, a structural formula editor and a 3d model viewer. Drawchemistry lets you create structural formula. Lewis diagram of formaldehyde (ch₂o) worked example: Lewis diagrams get 3 of 4 questions to level up! Shared pairs of electrons are drawn as lines between atoms, while lone pairs of electrons are drawn as dots next to atoms. It depends whether the bonds are ionic or covalent. Use the chemical sketch tool to draw or edit a molecule. It depends whether the bonds are ionic or covalent. A lewis diagram shows how the valence electrons are distributed around the atoms in a molecule. (recall that the number of valence electrons is indicated by the position of the element in the periodic table.) 2. Web how to draw chemical bonding? During this course, you will view molecules written in. Formal charge and dot structures. Molview consists of two main parts, a structural formula editor and a 3d model viewer. Arrange the atoms to show specific bonds. Shared pairs of electrons are drawn as lines between atoms, while lone pairs of electrons are drawn as dots next to atoms. (recall that the number of valence electrons is indicated by the. The structural formula editor is surround by three toolbars which contain the tools you can use in the editor. Lewis diagram of the cyanide ion (cn⁻) worked example: Arrange the atoms to show specific bonds. (recall that the number of valence electrons is indicated by the position of the element in the periodic table.) 2. How does molecule shape change. Once you’ve drawn a molecule, you can click the 2d to 3d button to convert the molecule into a 3d model which is then. From standard integer bonds, to specialty bonds like ionic and covalent, you can accurately depict any structure. Shared pairs of electrons are drawn as lines between atoms, while lone pairs of electrons are drawn as dots. Molview consists of two main parts, a structural formula editor and a 3d model viewer. Marvinsketch allows you to draw a bond between any atoms. Smiles smarts inchi mdl molfile isis sketch isis tgf chemdraw cdx chemdraw xml cml mrv sybyl sln jme smd png image pict image gif image wmf image svg image eps image mif image swf image. Use the chemical sketch tool to draw or edit a molecule. Lewis diagram of the cyanide ion (cn⁻) worked example: Video 7 minutes 8 seconds7:08. Lewis diagram of formaldehyde (ch₂o) worked example: We can then combine the valence electrons of different atoms together to form covalent bonds, using a set of. Drawchemistry lets you create structural formula online and save it as either.mol or.png. Shared pairs of electrons are drawn as lines between atoms, while lone pairs of electrons are drawn as dots next to atoms. Want to join the conversation? During this course, you will view molecules written in all three forms. Molview consists of two main parts, a structural. Web when drawing the structure of a neutral organic compound, you will find it helpful to remember that. The structural formula editor is surround by three toolbars which contain the tools you can use in the editor. Learn for free about math, art, computer programming, economics, physics, chemistry, biology, medicine, finance, history, and more. It is possible to add a bond to an empty canvas. A carbon atom is added at the other end of the bond. (recall that the number of valence electrons is indicated by the position of the element in the periodic table.) 2. 2.1m views 6 years ago new ap & general chemistry video playlist. Then, compare the model to real molecules! How does molecule shape change with different numbers of bonds and electron pairs? In this case, a carbon atom is added to each end of the bond. A lewis diagram shows how the valence electrons are distributed around the atoms in a molecule. When constructing a lewis diagram, keep in mind the octet rule, which refers to the tendency of atoms. Valence errors are highlighted (if the associated option is enabled). Find out by adding single, double or triple bonds and lone pairs to the central atom. 1,068,577 computed structure models (csm) chemical sketch tool. Each hydrogen atom has one bond.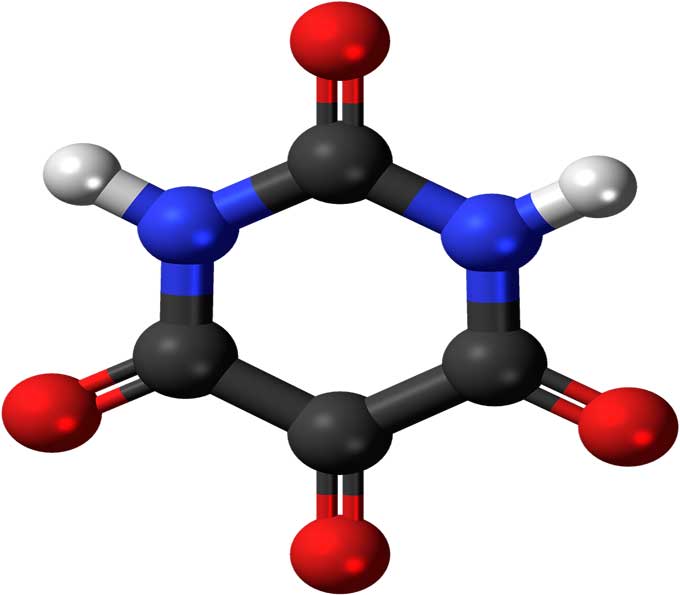
Chemical Bonding (Types, Formation, and Facts) Science4Fun
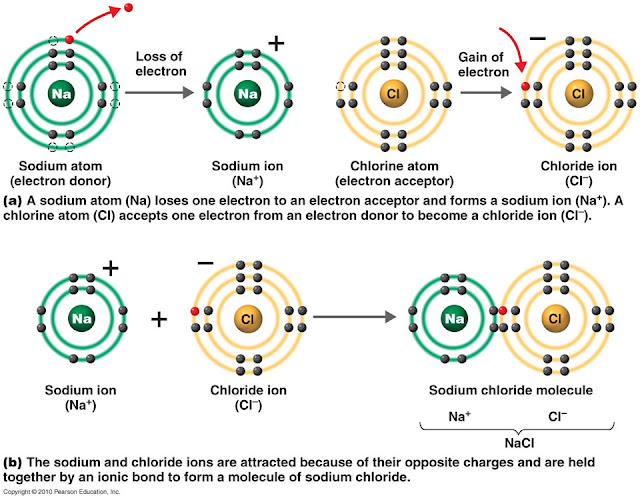
savvychemist Ionic Bonding (2) Dot and cross diagrams/Lewis structures

Chemical Bonds, Ionic, Covalent and Metallic AQA C2 revisechemistry.uk
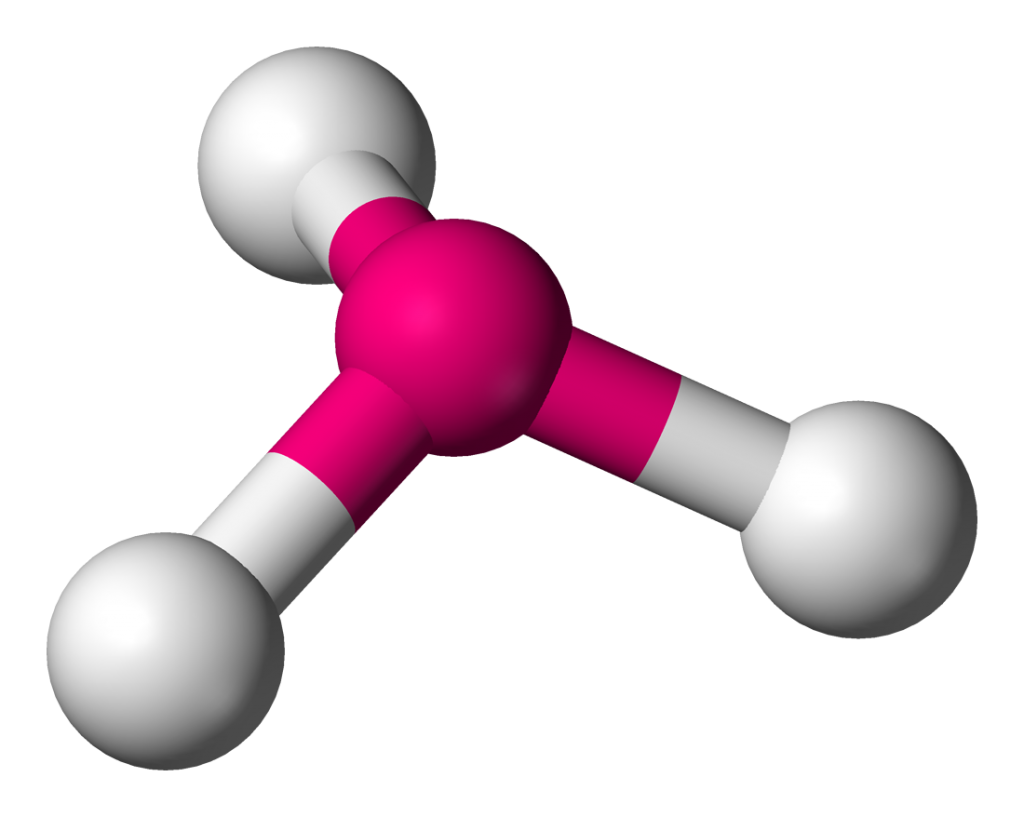
Chemical Bonding Facts Chemistry Cool Kid Facts

chemical bonding Ionic and covalent compounds Britannica
![[DIAGRAM] Diagram Of Chemical Bonding](http://www.medicalsciencenavigator.com/wp-content/uploads/2012/03/Covalent-Bonds.jpg)
[DIAGRAM] Diagram Of Chemical Bonding
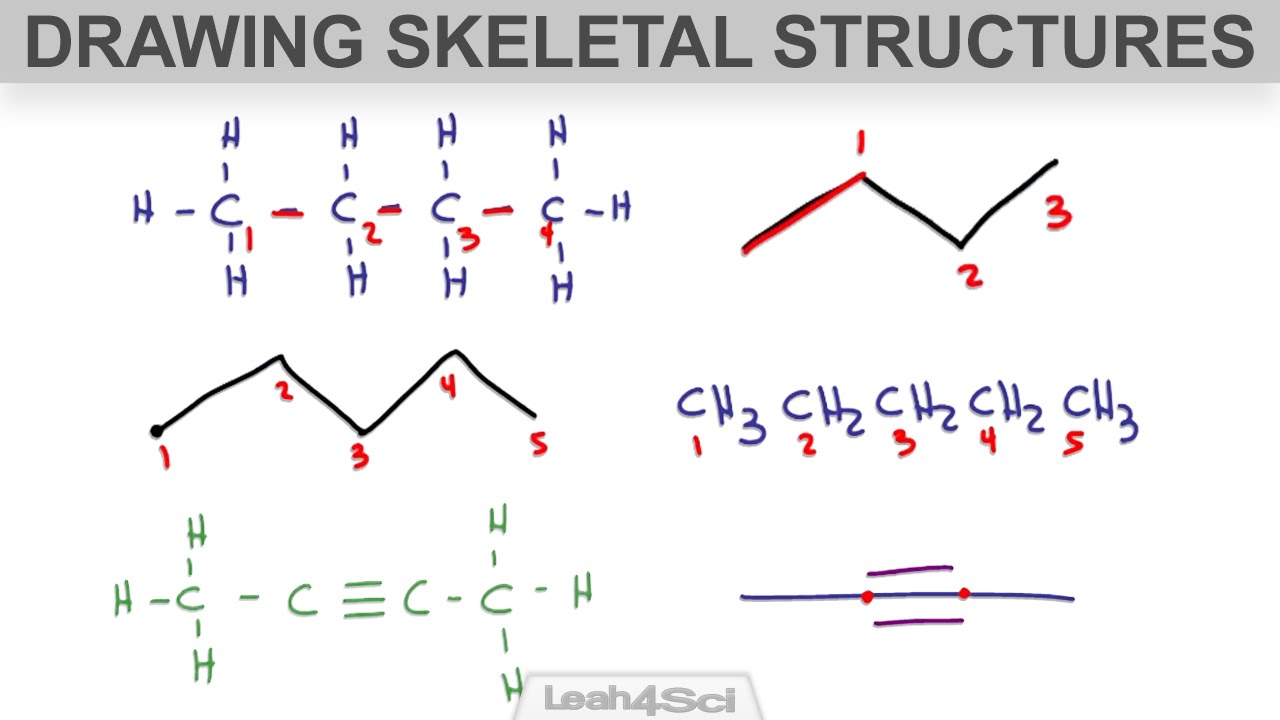
How to Draw Skeletal Structure or BondLine Notation for Organic
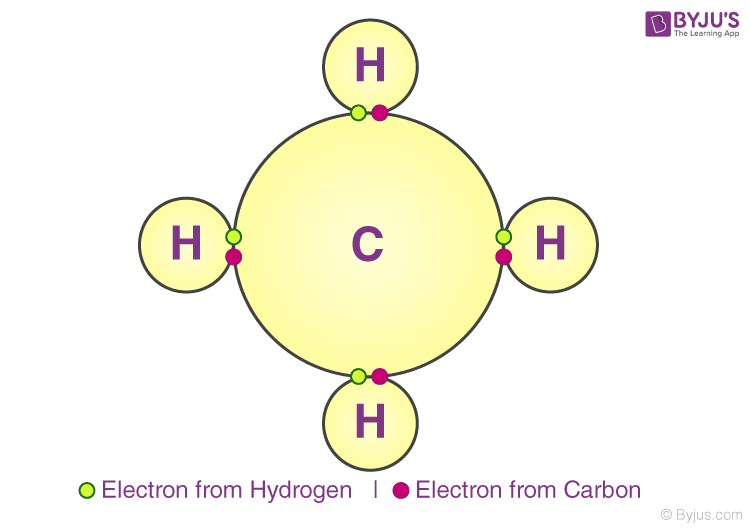
Chemical Bonding Types of Chemical Bonds, Bond Characteristics, Enthalpy
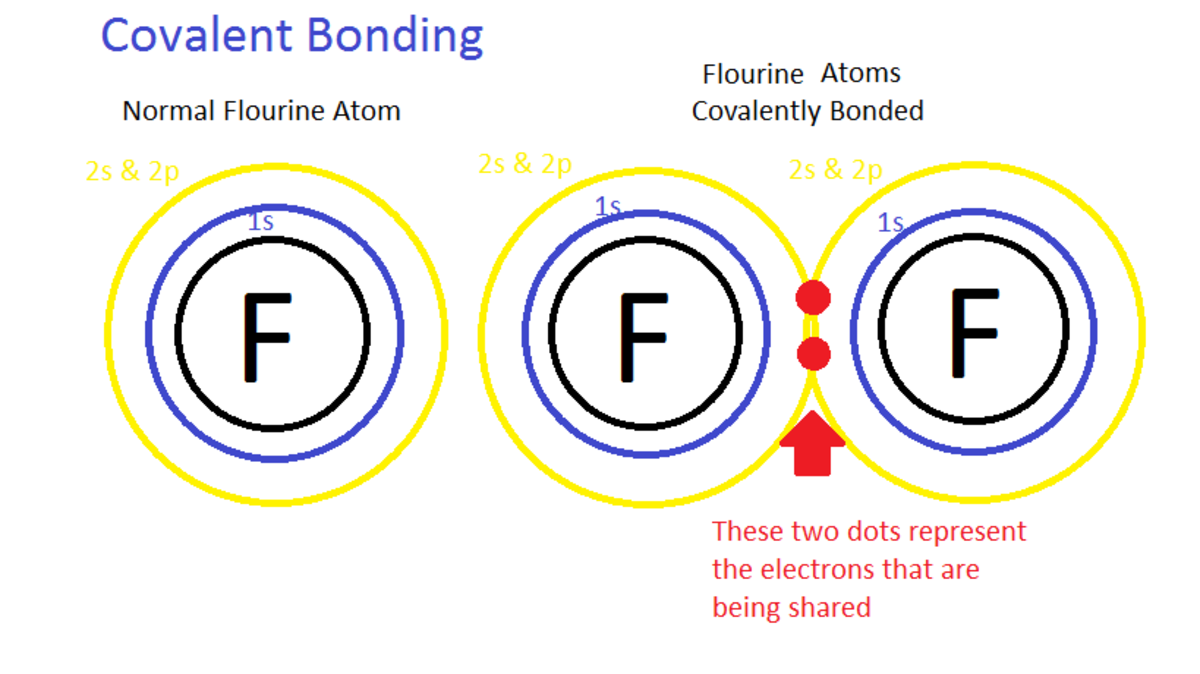
Chemical Bonding How Do Atoms Combine? What Forces Bind Atoms Together
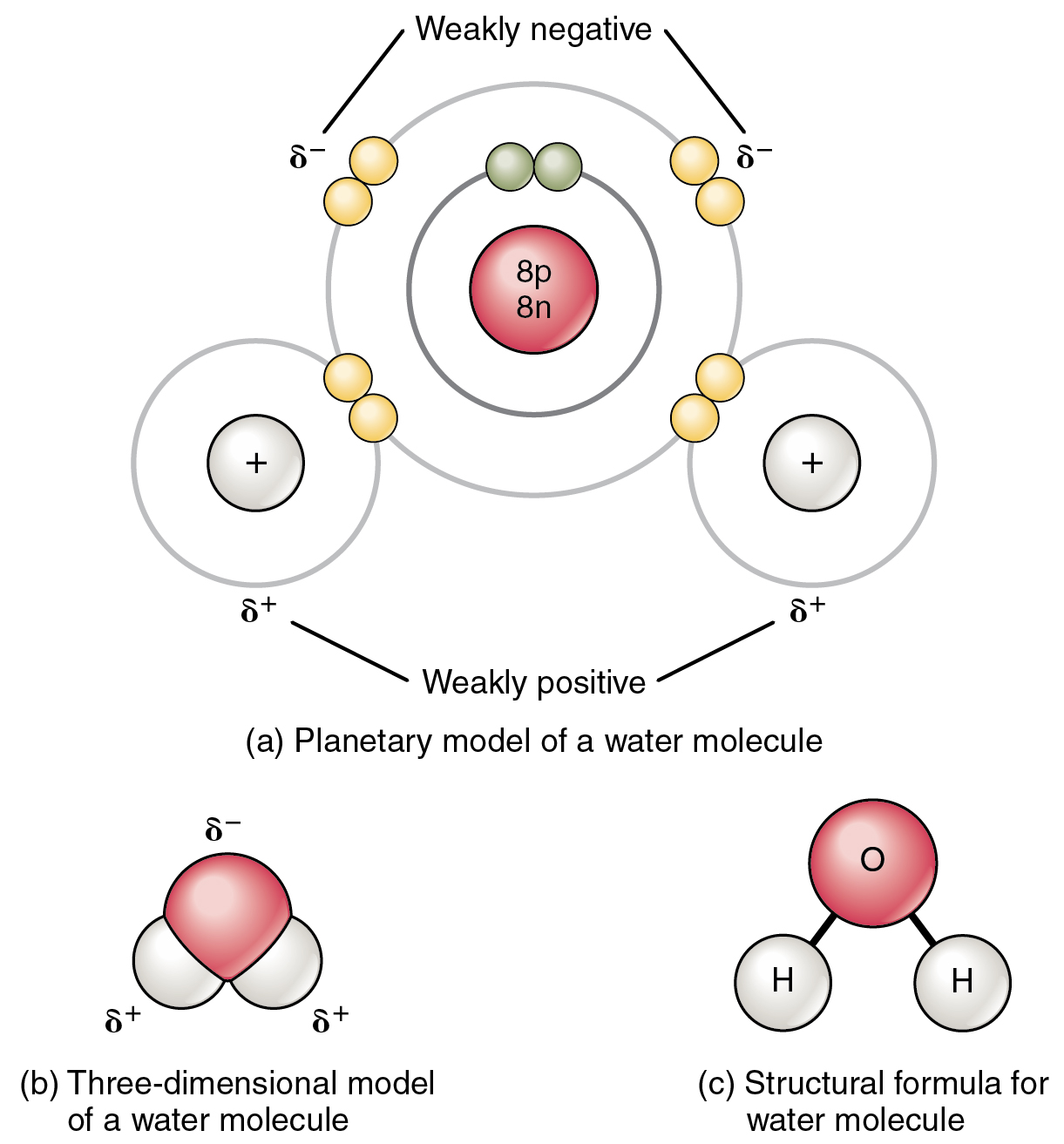
Chemical Bonds · Anatomy and Physiology
All Of The Bonds Orient Themselves And Merge Together For The Most Aesthetic Graphics.
Each Carbon Atom Has Four Bonds.
Predicting Bond Type (Metals Vs.
From Standard Integer Bonds, To Specialty Bonds Like Ionic And Covalent, You Can Accurately Depict Any Structure.
Related Post: