Ch4 Drawing
Ch4 Drawing - Methane is one of the simple organic molecules, given its straightforward structure. I also go over hybridization, shape. Next, a search of electrons is required by a single ch4 molecule to reach a stable condition. #2 next, indicate lone pairs on the atoms. The lewis structure is a highly simplified representation of the valence electrons in a chemical species such as an atom, ion, or molecule. Molecular orbital theory describes the distribution of electrons in molecular orbitals formed by the overlap of atomic orbitals. Draw resonance structures of some molecules. Firstly, look for the total number of valence electrons required by a single ch4 molecule, which is sixteen. Web 86k views 3 years ago. #1 draw a rough sketch of the structure. Find more chemistry widgets in wolfram|alpha. In methane, the carbon atom has four valence electrons, and each hydrogen atom has one valence electron. It has the chemical formula of ch4 and comprises one carbon atom forming bonds with four hydrogen atoms. (valence electrons are the electrons that are present in the outermost orbit of any. Web how to draw lewis. Count the total number of valence electrons. Ch4 lewis structure, hybridization, molecular geometry, bond angle and shape. Assign formal charge to an atom in a dot structure. Let’s break down each step in more detail. Drawing the lewis structure of ch 4 o is very simple. Video on lewis structure of ch4. It's one of the easier lewis structures to draw. It has the chemical formula of ch4 and comprises one carbon atom forming bonds with four hydrogen atoms. First, determine the total number of valence electrons. Web a video explanation of how to draw the lewis dot structure for methane, along with information about the. Web how to draw the lewis dot structure for ch4: Web 86k views 3 years ago. Assess the stability of a structure by considering formal charges of atoms. Send feedback | visit wolfram|alpha. Web drawing the lewis structure for ch 4 (named methane) requires only single bonds. The compound is one of the main constituents of natural gas. Find the total valence electrons in ch4 molecule. Let’s break down each step in more detail. I also go over hybridization, shape. Count the total number of valence electrons. This structure is also available as a 2d mol file or as a computed 3d sd file. For the ch4 structure use the periodic table to find. Understanding the molecular orbital diagram of ch4 provides insights into its bonding and stability. Web draw the lewis dot structure of a given molecule or ion. Web 86k views 3 years ago. Methane is one of the simple organic molecules, given its straightforward structure. Count the total number of valence electrons. Draw resonance structures of some molecules. The 3d structure may be viewed using java or javascript. The carbon atom (c) is at the center and it is surrounded by 4 hydrogen atoms (h). R 50 (refrigerant) permanent link for this species. Web you will be familiar with drawing methane, ch 4, using dots and crosses diagrams, but it is worth looking at its structure a bit more closely. Assign formal charge to an atom in a dot structure. I also go over hybridization, shape. Web drawing the lewis structure for ch 4 (named. Assess the stability of a structure by considering formal charges of atoms. Here’s how to do it: Web 86k views 3 years ago. Determine the total number of valence electrons in the molecule. Web to properly draw the ch 4 lewis structure, follow these steps: R 50 (refrigerant) permanent link for this species. Next, a search of electrons is required by a single ch4 molecule to reach a stable condition. Count the total number of valence electrons. Find the total valence electrons in ch4 molecule. Here’s how to do it: I quickly take you through how to draw the lewis structure of methane, ch4. Web to find the hybridization for ch4 we’ll first determine the steric number. Next, a search of electrons is required by a single ch4 molecule to reach a stable condition. Web lewis structure of ch4 (or methane) contains four single bonds between each carbon (c) and hydrogen (h) atoms. Web ch4, also known as methane, is a covalent compound consisting of one carbon atom bonded to four hydrogen atoms. Web drawing the lewis structure for ch 4 (named methane) requires only single bonds. Let’s walk through the process: It has the chemical formula of ch4 and comprises one carbon atom forming bonds with four hydrogen atoms. Web here we will learn about how the lewis dot structure is drawn for ch4 molecule, step by step. Number of steps can be changed according the complexity of the molecule or ion. Drawing the lewis structure for ch4. Find the total valence electrons in ch4 molecule. Drawing the lewis structure of ch 4 o is very simple. Determine the total number of valence electrons in the molecule. Let’s break down each step in more detail. Web you will be familiar with drawing methane, ch 4, using dots and crosses diagrams, but it is worth looking at its structure a bit more closely.
Lewis Dot Diagram Ch4
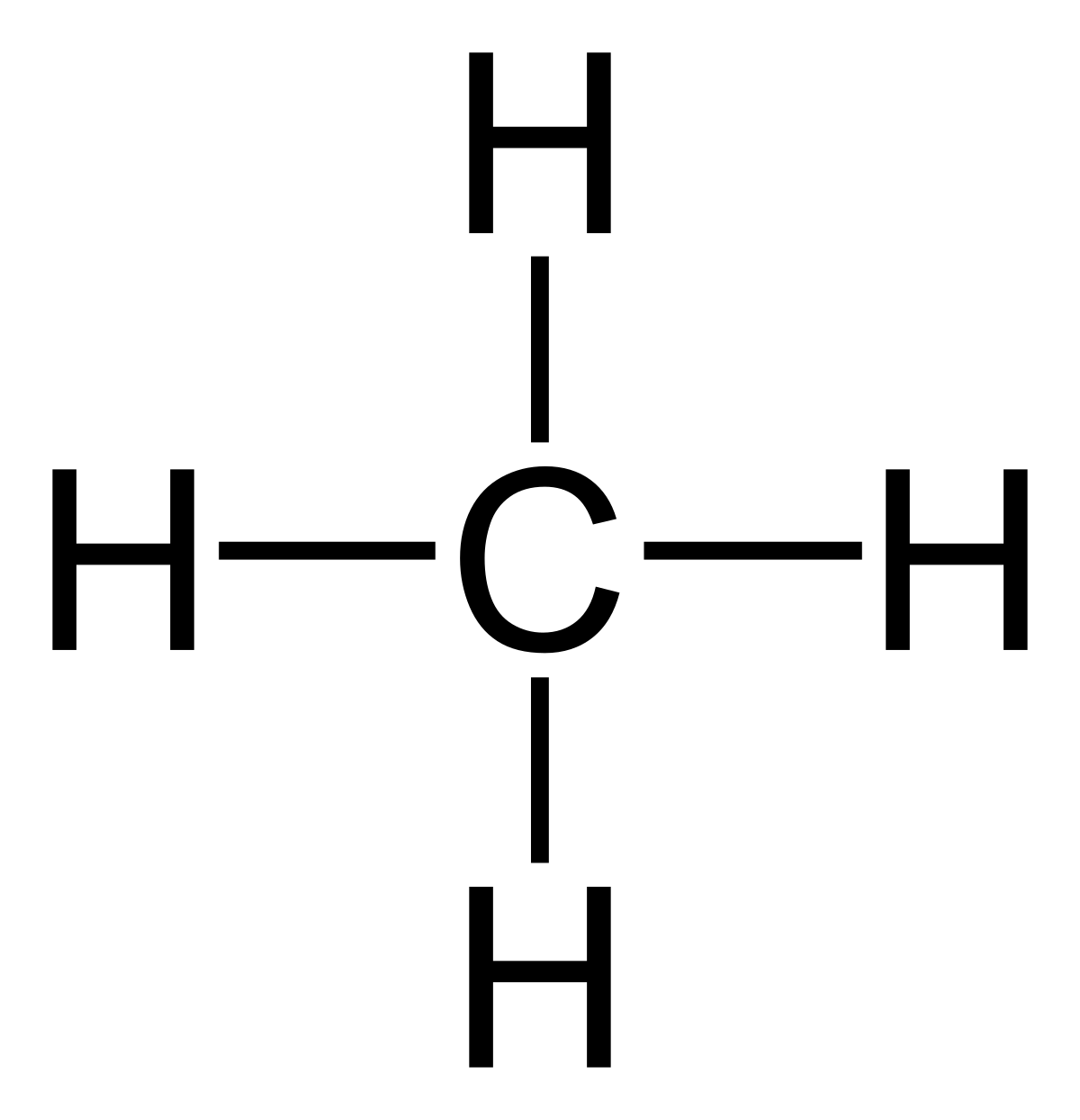
Dot Diagram For Ch4

How to Draw the Lewis Dot Structure for CH4 Methane YouTube

Electron Dot Diagram For Methane

How to draw CH4 Lewis Structure? Science Education and Tutorials
Methane Molecule CH4 Drawing Drawing by Frank Ramspott Fine Art America
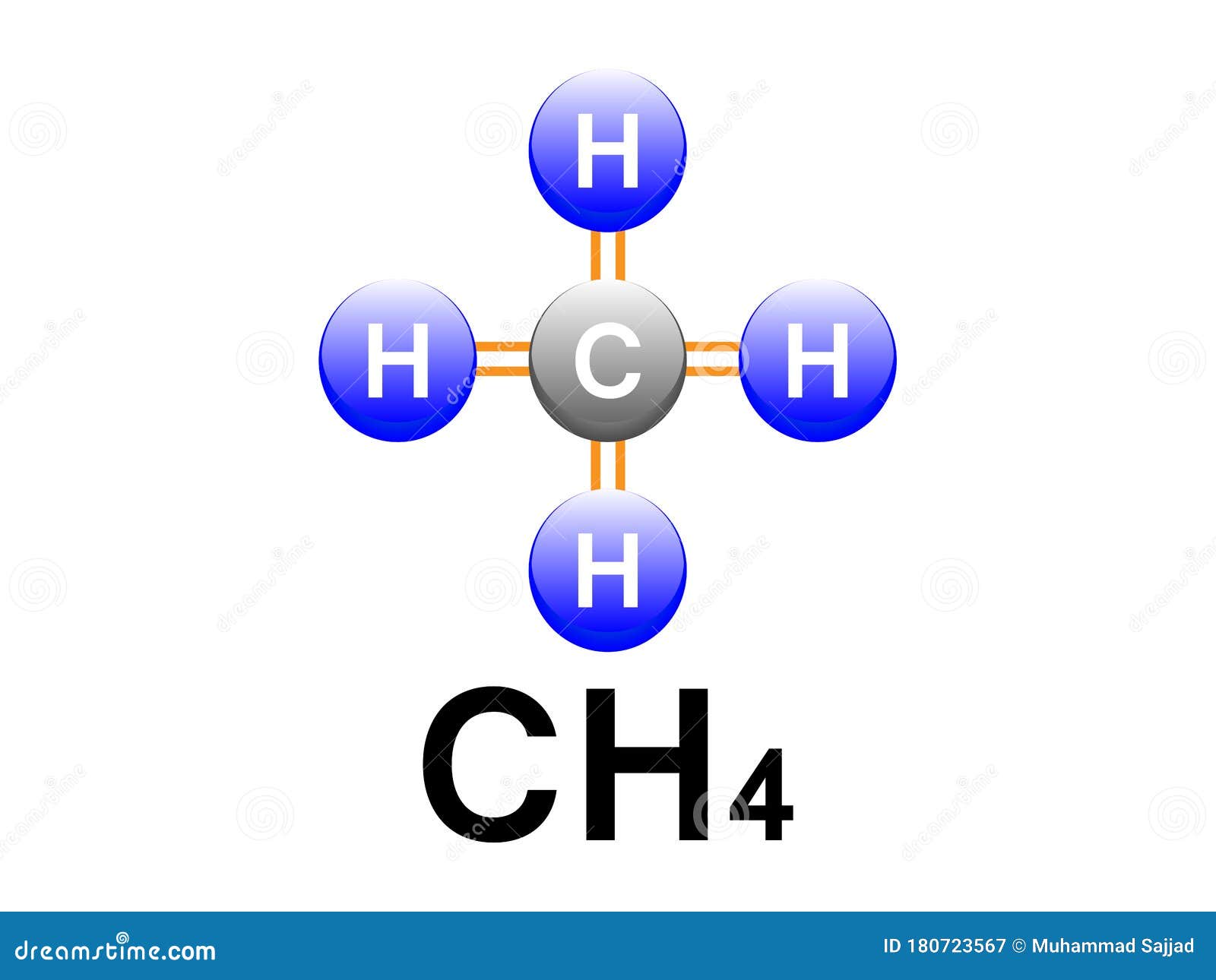
CH4 Methane Covalent Bonding .Methane Formula Diagram Design for

Draw Lewis Structure For Ch4 Nelson Tardwilis

How to draw CH4 Lewis Structure? Science Education and Tutorials

CH4 Lewis Structure, Molecular Geometry, and Hybridization
First, Determine The Total Number Of Valence Electrons.
#2 Next, Indicate Lone Pairs On The Atoms.
Methane Is The Simplest Of Saturated Hydrocarbons With A Chemical Formula Ch 4.
R 50 (Refrigerant) Permanent Link For This Species.
Related Post: